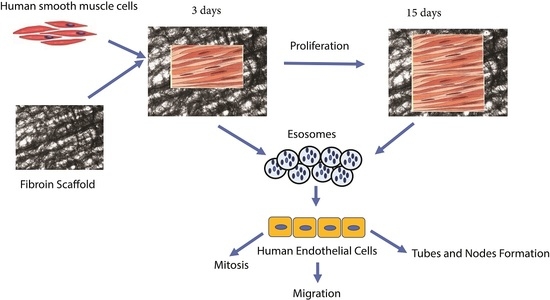
Adult Human Vascular Smooth Muscle Cells on 3D Silk Fibroin Nonwovens Release Exosomes Enriched in Angiogenic and Growth-Promoting Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. C/HE-3D-SFnws
2.2. C/HE-3D-SFnws Samples for In Vitro Cell Cultures
2.3. Cells
2.4. Intravital AHSMCs Staining and In Vitro Culture
2.5. AHSMCs’ Total DNA Assay
2.6. Assay of D-glucose Consumption
2.7. Phoshoproteins Array Analysis
2.8. Isolation, Characterization, and Quantification of Exosomes
2.9. AGFs Carried by AHSMC-Released Exosomes
2.10. In Vitro HECs’ Cultures
2.11. In Vitro HECs Migration Test
2.12. In Vitro HECs Tubes and Nodes Formation Assay
2.13. Statistical Analysis
3. Results
3.1. Carded/Hydroentangled (C/HE)-3D-SFnws
3.2. Growth and Metabolism of AHSMCs on C/HE-3D-SFnws vs. Polystyrene
3.3. Signalling Pathways Activated in C/HE-3D-SFnws- vs. Polystyrene-Attached AHSMCs
3.3.1. TGF-β Signalling Pathway
3.3.2. NF-κB Signalling Pathway
3.4. AGFs Released via Exosomes from AHSMCs Grown on C/HE-3D-SFnws vs. Polystyrene
3.5. Exosomes from C/HE-3D-SFnws-Stuck AHSMCs Powerfully Stimulate HECs to Grow, Migrate, and Make Endothelial Tubes/Nodes
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D-SFnws | three-dimensional silk fibroin nonwovens |
| AGFs | angiogenic and growth factors |
| AHSMCs | adult human smooth muscle cells |
| Akt | protein kinase B or PKB |
| ANGPT-1/2 | angiopoietin-1/2 |
| ANOVA | analysis of variance |
| ATM | ataxia-telangiectasia mutated Ser/Thr kinase |
| bFGF | basic Fibroblast Growth factor |
| c-Fos | component of AP1 transcription factors |
| c-Jun | component of AP1 transcription factors |
| CREB | cyclic AMP response element binding protein |
| C/HE | carding/hydroentanglement |
| C/N | carding/needling |
| DiOC18(3) | (3,3′-Dioctadecyl oxacarbocyanine perchlorate or DiO |
| (ds)DNA | double strand DNA |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| eIF-2α | eukaryotic translation initiation factor-2α |
| ELISA | enzyme-linked immunosorbent assay |
| FA | formic acid |
| FBS | foetal bovine serum |
| GM-CFS | granulocyte macrophage-colony stimulating factor |
| GRO-α/-β/-γ | growth-regulated oncogene-α/-β/-γ (or CXCL1/2/3) |
| HADAC | histone deacetylase |
| HDFs | human dermal fibroblasts |
| HECs | human ECs |
| IKK | IkappaB kinase |
| IκBα | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IL- | interleukin- |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 (or CCL2) |
| MMP | matrix metalloprotease |
| MSK-1 | mitogen, and stress-activated protein kinase |
| NF-κB | nuclear factor κB |
| rh | recombinant human |
| SD | standard deviation |
| SF | silk fibroin |
| SMAD | small mother against decapentaplegic homolog |
| SMCs | smooth muscle cells |
| TAK-1 | TGF-β-activated kinase-1 |
| TCF/LEF | T-cell factor/Lymphoid enhancer factor |
| TGF-β | transforming growth factor-beta |
| Tie-2 | ANGPT-1 receptor |
| TIMP-1/2 | tissue inhibitor of metalloproteinases-1/2 |
| TLR | Toll-like receptor |
| uPA | urokinase-like plasminogen activator |
| uPAR | uPA surface receptor |
| VEGF | vascular endothelial growth factor |
| VEGF-R | VEGF receptor |
References
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural Comparison of Various Silkworm Silks: An Insight into the Structure-Property Relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Kaewpirom, S.; Boonsang, S. Influence of alcohol treatments on properties of silk-fibroin-based films for highly optocally transparent coating applications. RSC Adv. 2020, 10, 15913–15923. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yang, L.; Zeng, Y.; Gao, X.; Xu, H. Poly(ethylene glycol)-modified silk fibroin membrane as a carrier for limbal epithelial stem cell transplantation in a rabbit LSCD model. Stem Cell Res. Ther. 2017, 8, 256. [Google Scholar] [CrossRef]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Chen, Y.; Wang, J.; Chen, Y.; Mao, C.; Yang, M. Polydopamine modification of silk fibroin membranes significantly promotes their wound healing effect. Biomater. Sci. 2019, 7, 5232–5237. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Dal Prà, I.; Freddi, G.; Minic, J.; Chiarini, A.; Armato, U. De novo engineering of reticular connective tissue in vivo by silk fibroin nonwoven materials. Biomaterials 2005, 26, 1987–1999. [Google Scholar] [CrossRef]
- Chiarini, A.; Freddi, G.; Liu, D.; Armato, U.; Dal Prà, I. Biocompatible silk noil-based three-dimensional carded-needled nonwoven scaffolds guide the engineering of novel skin connective tissue. Tissue Eng. Part A 2016, 22, 1047–1060. [Google Scholar] [CrossRef]
- Armato, U.; Dal Pra, I.; Chiarini, A.; Freddi, G. Will silk fibroin nanofiber scaffolds ever hold a useful place in Translational Regenerative Medicine? Int. J. Burns Trauma 2011, 1, 27–33. [Google Scholar]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- ISO and CEN Definition of Nonwovens. Available online: https://www.edana.org/docs/default-source/edana-nonwovens/iso-and-cen-definition-of-nonwovens.pdf?sfvrsn=21822973_221 (accessed on 10 January 2022).
- Dal Prà, I.; Chiarini, A.; Boschi, A.; Freddi, G.; Armato, U. Novel dermo-epidermal equivalents on silk fibroin-based formic acid-crosslinked three-dimensional nonwoven devices with prospective applications in human tissue engineering/regeneration/repair. Int. J. Mol. Med. 2006, 18, 241–247. [Google Scholar] [CrossRef]
- Hu, P.; Chiarini, A.; Wu, J.; Freddi, G.; Nie, K.; Armato, U.; Dal Prà, I. Exosomes of adult human fibroblasts cultured on 3D silk fibroin nonwovens intensely stimulate neoangiogenesis. Burns Trauma 2021, 9, tkab003. [Google Scholar] [CrossRef]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts and enhance angiogenesis in vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Baruah, J.; Wary, K.K. Exosomes in the Regulation of Vascular Endothelial Cell Regeneration. Front. Cell. Dev. Biol. 2020, 7, 353. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.F.; Zhu, H.; Millard, R.W.; Fan, G.C. Exosomes Function in Pro- and Anti-Angiogenesis. Curr. Angiogenes 2013, 2, 54–59. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Poon, I. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, A.; Szuścik, I.; Kuśnierz-Cabala, B.; Kapusta, M.; Konkolewska, M.; Żurakowski, A.; Georgescu, A.; Stępień, E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Cracov. 2015, 55, 35–48. [Google Scholar]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Yang, Q.; Wang, Q.; Shi, C.; Wang, D.; Armato, U.; Dal Prà, I.; Chiarini, A. Mesenchymal stromal cells-exosomes: A promising cell-free therapeutic tool for wound healing and cutaneous regeneration. Burns Trauma 2019, 7, 38. [Google Scholar] [CrossRef]
- Barnes, B.J.; Somerville, C.C. Modulating cytokine production via select packaging and secretion from extracellular vesicles. Front. Immunol. 2020, 11, 1040. [Google Scholar] [CrossRef]
- Mikawa, T.; Gourdie, R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 1996, 174, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Gadson, P.F., Jr.; Dalton, M.L.; Patterson, E.; Svoboda, D.D.; Hutchinson, L.; Schram, D.; Rosenquist, T.H. Differential response of mesoderm- and neural crest-derived smooth muscle to TGF-β1: Regulation of c-myb and α1 (I) procollagen genes. Exp. Cell Res. 1997, 230, 169–180. [Google Scholar] [CrossRef]
- Topouzis, S.; Majesky, M.W. Smooth muscle lineage diversity in the chick embryo: Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-β. Dev. Biol. 1996, 178, 430–445. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell Mol. Life Sci. 2014, 71, 2271–2288. [Google Scholar] [CrossRef] [Green Version]
- DeRuiter, M.C.; Poelmann, R.E.; VanMunsteren, J.C.; Mironov, V.; Markwald, R.R.; Gittenberger-de Groot, A.C. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ. Res. 1997, 80, 444–451. [Google Scholar] [CrossRef]
- Iyemere, V.P.; Proudfoot, D.; Weissberg, P.L.; Shanahan, C.M. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 2006, 260, 192–210. [Google Scholar] [CrossRef]
- Olson, L.E.; Soriano, P. PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev. Cell. 2011, 20, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Rensen, S.S.; Doevendans, P.A.; van Eys, G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Bargehr, J.; Low, L.; Cheung, C.; Bernard, W.G.; Iyer, D.; Bennett, M.R.; Gambardella, L.; Sinha, S. Embryological Origin of Human Smooth Muscle Cells Influences Their Ability to Support Endothelial Network Formation. Stem Cells Transl. Med. 2016, 5, 946–959. [Google Scholar] [CrossRef] [Green Version]
- Alessandrino, A.; Chiarini, A.; Biagiotti, M.; Dal Prà, I.; Bassani, G.A.; Vincoli, V.; Settembrini, P.; Pierimarchi, P.; Freddi, G.; Armato, U. Three-Layered Silk Fibroin Tubular Scaffold for the Repair and Regeneration of Small Caliber Blood Vessels: From Design to in vivo Pilot Tests. Front. Bioeng. Biotechnol. 2019, 7, 356. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukuda, D.; Higashikuni, Y.; Hirata, Y.; Komuro, I.; Saotome, T.; Yamashita, Y.; Asakura, T.; Sata, M. Biodegradable Extremely-Small-Diameter Vascular Graft Made of Silk Fibroin can be Implanted in Mice. J. Atheroscler Thromb. 2020, 27, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Gays, D.; Hess, C.; Camporeale, A.; Ala, U.; Provero, P.; Mosimann, C.; Santoro, M.M. An exclusive cellular and molecular network governs intestinal smooth muscle cell differentiation in vertebrates. Development 2017, 144, 464–478. [Google Scholar] [CrossRef] [Green Version]
- Yap, H.M.; Israf, D.A.; Harith, H.H.; Tham, C.L.; Sulaiman, M.R. Crosstalk between Signaling Pathways Involved in the Regulation of Airway Smooth Muscle Cell Hyperplasia. Front. Pharmacol. 2019, 10, 1148. [Google Scholar] [CrossRef]
- Oszajca, K.; Szemraj, J. Assessment of the correlation between oxidative stress and expression of MMP-2, TIMP-1 and COX-2 in human aortic smooth muscle cells. Arch. Med. Sci. Atheroscler. Dis. 2021, 6, e158–e165. [Google Scholar] [CrossRef]
- Clifford, R.L.; John, A.E.; Brightling, C.E.; Knox, A.J. Abnormal histone methylation is responsible for increased vascular endothelial growth factor 165a secretion from airway smooth muscle cells in asthma. J. Immunol. 2012, 189, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Clauser, S.; Meilhac, O.; Bièche, I.; Raynal, P.; Bruneval, P.; Michel, J.B.; Borgel, D. Increased secretion of Gas6 by smooth muscle cells in human atherosclerotic carotid plaques. Thromb. Haemost. 2012, 107, 140–149. [Google Scholar] [CrossRef]
- Alagappan, V.K.; McKay, S.; Widyastuti, A.; Garrelds, I.M.; Bogers, A.J.; Hoogsteden, H.C.; Hirst, S.J.; Sharma, H.S. Proinflammatory cytokines upregulate mRNA expression and secretion of vascular endothelial growth factor in cultured human airway smooth muscle cells. Cell Biochem. Biophys. 2005, 43, 119–129. [Google Scholar] [CrossRef]
- Feng, P.H.; Hsiung, T.C.; Kuo, H.P.; Huang, C.D. Cross-talk between bradykinin and epidermal growth factor in regulating IL-6 production in human airway smooth muscle cells. Chang Gung Med. J. 2010, 33, 92–99. [Google Scholar]
- Seol, H.J.; Oh, M.J.; Kim, H.J. Endothelin-1 expression by vascular endothelial growth factor in human umbilical vein endothelial cells and aortic smooth muscle cells. Hypertens. Pregnancy 2011, 30, 295–301. [Google Scholar] [CrossRef]
- Comelli, L.; Rocchiccioli, S.; Smirni, S.; Salvetti, A.; Signore, G.; Citti, L.; Trivella, M.G.; Cecchettini, A. Characterization of secreted vesicles from vascular smooth muscle cells. Mol. Biosyst. 2014, 10, 1146–1152. [Google Scholar] [CrossRef]
- Neradil, J.; Kyr, M.; Polaskova, K.; Kren, L.; Macigova, P.; Skoda, J.; Sterba, J.; Veselska, R. Phospho-Protein Arrays as Effective Tools for Screening Possible Targets for Kinase Inhibitors and Their Use in Precision Pediatric Oncology. Front. Oncol. 2019, 9, 930. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [Green Version]
- Kapustin, A.N.; Chatrou, M.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue Assay for Cellular Growth and Viability In Vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Armato, U.; Romano, F.; Andreis, P.G.; Paccagnella, L.; Marchesini, C. Growth stimulation and apoptosis induced in cultures of neonatal rat liver cells by repeated exposures to epidermal growth factor/urogastrone with or without associated pancreatic hormones. Cell Tissue Res. 1986, 245, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A. Biomechanics of soft tissue. In Handbook of Materials Behavior Models; Lemaitre, J., Ed.; Section 10.11; Academic Press: Cambridge, MS, USA, 2001; pp. 1057–1071. ISBN 978-0-12-443341-0. [Google Scholar]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Jiang, W.; Yang, J.; Mao, Y.Q.; Zhang, Y.; Yang, W.; Yang, D.; Burkholder, B.; Huang, R.F.; Huang, R.P. A biotin label-based antibody array for high-content profiling of protein expression. Cancer Genom. Proteom. 2010, 7, 129–141. [Google Scholar]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef] [Green Version]
- Raines, E.W.; Dower, S.K.; Ross, R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science 1989, 243, 393–396. [Google Scholar] [CrossRef]
- Gay, C.G.; Winkles, J.A. Interleukin 1 regulates heparin-binding growth factor 2 gene expression in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 1991, 88, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Beasley, D.; Cooper, A.L. Constitutive expression of interleukin-1α precursor promotes human vascular smooth muscle cell proliferation. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H901–H912. [Google Scholar] [CrossRef]
- Downie, G.H.; Ryan, U.S.; Hayes, B.A.; Friedman, M. Interleukin-2 directly increases albumin permeability of bovine and human vascular endothelium in vitro. Am. J. Respir. Cell Mol. Biol. 1992, 7, 58–65. [Google Scholar] [CrossRef]
- Bae, J.; Park, D.; Lee, Y.S.; Young, D. Interleukin-2 promotes angiogenesis by activation of Akt and increase of ROS. J. Microbiol. Biotechnol. 2008, 18, 377–382. [Google Scholar] [PubMed]
- Nabata, T.; Fukuo, K.; Morimoto, S.; Kitano, S.; Momose, N.; Hirotani, A.; Nakahashi, T.; Nishibe, A.; Hata, S.; Niinobu, T.; et al. Interleukin-2 modulates the responsiveness to angiotensin II in cultured vascular smooth muscle cells. Atherosclerosis 1997, 133, 23–30. [Google Scholar] [CrossRef]
- Schultz, K.; Murthy, V.; Tatro, J.B.; Beasley, D. Endogenous interleukin-1α promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2927–H2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.W.; Eum, S.Y.; Chen, K.C.; Hennig, B.; Toborek, M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol. Med. 2004, 10, 19–27. [Google Scholar] [CrossRef]
- Skaria, T.; Burgener, J.; Bachli, E.; Schoedon, G. IL-4 Causes Hyperpermeability of Vascular Endothelial Cells through Wnt5A Signaling. PLoS ONE 2016, 11, e0156002. [Google Scholar] [CrossRef] [Green Version]
- Klein, N.J.; Rigley, K.P.; Callard, R.E. IL-4 regulates the morphology, cytoskeleton, and proliferation of human umbilical vein endothelial cells: Relationship between vimentin and CD23. Int. Immunol. 1993, 5, 293. [Google Scholar] [CrossRef]
- Toi, M.; Harris, A.L.; Bicknell, R. Interleukin-4 is a potent mitogen for capillary endothelium. Biochem. Biophys. Res. Commun. 1991, 174, 1287–1293. [Google Scholar] [CrossRef]
- Fukushi, J.; Morisaki, T.; Shono, T.; Nishie, A.; Torisu, H.; Ono, M.; Kuwano, M. Novel biological functions of interleukin-4: Formation of tube-like structures by vascular endothelial cells in vitro and angiogenesis in vivo. Biochem. Biophys. Res. Commun. 1998, 250, 444–448. [Google Scholar] [CrossRef]
- Ikeda, U.; Ikeda, M.; Oohara, T.; Oguchi, A.; Kamitani, T.; Tsuruya, Y.; Kano, S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am. J. Physiol. 1991, 260, H1713–H1717. [Google Scholar] [CrossRef]
- Ljungberg, L.U.; Zegeye, M.M.; Kardeby, C.; Fälker, K.; Repsilber, D.; Sirsjö, A. Global Transcriptional Profiling Reveals Novel Autocrine Functions of Interleukin 6 in Human Vascular Endothelial Cells. Mediators Inflamm. 2020, 2020, 4623107. [Google Scholar] [CrossRef]
- Keeley, E.C.; Mehrad, B.; Strieter, R.M. Chemokines as mediators of neovascularization. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1928–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, R.J.; Hansen, H.K.; Christensen, L.P. Temporally expressed PDGF and FGF-2 regulate embryonic coronary artery formation and growth. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Holnthoner, W.; Pillinger, M.; Groger, M.; Wolff, K.; Ashton, A.W.; Albanese, C.; Neumeister, P.; Pestell, R.G.; Petzelbauer, P. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J. Biol. Chem. 2002, 277, 45847–45853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, Y.; Mou, Y.; Zhao, Y.; Blankesteijn, W.M.; Hall, J.L. A role for the β-catenin/T-cell factor signaling cascade in vascular remodeling. Circ. Res. 2002, 90, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Krubasik, D.; Eisenach, P.A.; Kunz-Schughart, L.A. Granulocyte-macrophage colony stimulating factor induces endothelial capillary formation through induction of membrane-type 1 matrix metalloproteinase expression in vitro. Int. J. Cancer 2008, 122, 1261–1272. [Google Scholar] [CrossRef]
- Bussolino, F.; Ziche, M.; Wang, J.M.; Alessi, D.; Morbidelli, L.; Cremona, O.; Bosia, A.; Marchisio, P.C.; Mantovani, A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J. Clin. Investig. 1991, 87, 986–995. [Google Scholar] [CrossRef]
- Buschmann, I.R.; Busch, H.J.; Mies, G.; Hossmann, K.A. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation 2003, 108, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Frismantiene, A.; Philippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Ropraz, P.; Gabbiani, F.; Gabbiani, G. Phenotypic heterogeneity of rat arterial smooth muscle cell clones: Implications for the development of experimental intimal thickening. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Bochaton-Piallat, M.L.; Gabbiani, F.; Redard, M.; Desmoulière, A.; Gabbiani, G. Apoptosis takes part in cellularity regulation during rat intimal thickening. Am. J. Pathol. 1995, 146, 1059. [Google Scholar] [PubMed]
- Slomp, J.; Gittenberger-de Groot, A.C.; Glukhova, M.A.; van Munsteren, J.C.; Kockx, M.M.; Schwartz, S.M.; Koteliansky, V.E. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M.; Majesky, M.W.; Murry, C.E. The intima: Development and monoclonal responses to injury. Atherosclerosis 1995, 118, S125–S140. [Google Scholar] [CrossRef]
- Campbell, J.H.; Campbell, G.R. Smooth muscle phenotypic modulation—A personal experience. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1784–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamley-Campbell, J.; Campbell, G.R.; Ross, R. The smooth muscle cell in culture. Physiol. Rev. 1979, 59, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruemmer, D.; Daugherty, A.; Lu, H.; Rateri, D.L. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann. N. Y. Acad. Sci. 2011, 1245, 7–10. [Google Scholar] [CrossRef]
- Chiarini, A.; Petrini, P.; Bozzini, S.; Dal Pra, I.; Armato, U. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: In vitro interactions with human cells. Biomaterials 2003, 24, 789–799. [Google Scholar] [CrossRef]
- Tan, J.Y.; Wen, J.C.; Shi, W.H.; He, Q.; Zhu, L.; Liang, K.; Shao, Z.Z.; Yu, B. Effect of microtopographic structures of silk fibroin on endothelial cell behavior. Mol. Med. Rep. 2013, 7, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca-Cusachs, P.; Alcaraz, J.; Sunyer, R.; Samitier, J.; Farré, R.; Navajas, D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys. J. 2008, 94, 4984–4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamish, J.A.; He, P.; Kottke-Marchant, K.; Marchant, R.E. Molecular regulation of contractile smooth muscle cell phenotype: Implications for vascular tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 467–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Moses, J.C.; Mandal, B.B. Surface Patterning and Innate Physicochemical Attributes of Silk Films Concomitantly Govern Vascular Cell Dynamics. ACS Biomater. Sci. Eng. 2019, 5, 933–949. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 18, a022061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.R.; Lei, C.Q. TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses. Front. Immunol. 2021, 11, 608976. [Google Scholar] [CrossRef]
- Ouyang, C.; Nie, L.; Gu, M.; Wu, A.; Han, X.; Wang, X.; Shao, J.; Xia, Z. Transforming growth factor (TGF)-β-activated kinase 1 (TAK1) activation requires phosphorylation of serine 412 by protein kinase A catalytic subunit α (PKACalpha) and X-linked protein kinase (PRKX). J. Biol. Chem. 2014, 289, 24226–24237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, P.; Perrault, R.; Louis, S.; Zahradka, P. The cyclic AMP response element-binding protein (CREB) mediates smooth muscle cell proliferation in response to angiotensin II. J. Cell Commun. Signal. 2014, 8, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.C.; Randall, R.A.; Hill, C.S. Transforming growth factor β-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell. Biol. 2008, 28, 6889–6902. [Google Scholar] [CrossRef] [Green Version]
- Roelen, B.A.; Cohen, O.S.; Raychowdhury, M.K.; Chadee, D.N.; Zhang, Y.; Kyriakis, J.M.; Alessandrini, A.A.; Lin, H.Y. Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-β-induced nuclear accumulation. Am. J. Physiol. Cell Physiol. 2003, 285, C823–C830. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.A.; De La Torre, C.; Arnold, C.; Kohlhaas, J.; Kappert, L.; Hecker, M.; Feldner, A.; Korff, T. Assembly of vascular smooth muscle cells in 3D aggregates provokes cellular quiescence. Exp. Cell. Res. 2020, 388, 111782. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Harada, J.; Tashiro, S.; Gotoh-Mandeville, R.; Maekawa, T.; Ishii, S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem. 1999, 274, 8949–8957. [Google Scholar] [CrossRef] [Green Version]
- Verrecchia, F.; Tacheau, C.; Schorpp-Kistner, M.; Angel, P.; Mauviel, A. Induction of the AP-1 members c-Jun and JunB by TGF-β/Smad suppresses early Smad-driven gene activation. Oncogene 2001, 20, 2205–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H.; Xiao, C.; Paschal, A.E.; Bailey, S.T.; Rao, P.; Hayden, M.S.; Lee, K.Y.; Bussey, C.; Steckel, M.; Tanaka, N.; et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005, 19, 2668–2681. [Google Scholar] [CrossRef] [Green Version]
- Takaesu, G.; Surabhi, R.M.; Park, K.J.; Ninomiya-Tsuji, J.; Matsumoto, K.; Gaynor, R.B. TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J. Mol. Biol. 2003, 326, 105–115. [Google Scholar] [CrossRef]
- Dai, L.; Aye Thu, C.; Liu, X.Y.; Xi, J.; Cheung, P.C. TAK1, more than just innate immunity. IUBMB Life 2012, 64, 825–834. [Google Scholar] [CrossRef]
- Schmid, J.A.; Birbach, A. IκB kinase β (IKKβ/IKK2/IKBKB)—A key molecule in signaling to the transcription factor NF-κB. Cytokine Growth Factor Rev. 2008, 19, 157–165. [Google Scholar] [CrossRef]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-κB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.D.; Harrison, S.C. Structure of an IκBα/NF-κB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Chen, Y.; Yang, Z.; You, B.; Ruan, Y.C.; Peng, Y. Epidermal CFTR suppresses MAPK/NF-κB to promote cutaneous wound healing. Cell. Physiol. Biochem. 2016, 39, 2262–2274. [Google Scholar] [CrossRef]
- McKinnon, P.J. ATM and ataxia telangiectasia. EMBO Rep. 2004, 5, 772–776. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Paull, T.T. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005, 308, 551–554, Erratum in Science 2005, 308, 1870. [Google Scholar] [CrossRef]
- Lee, J.H.; Paull, T.T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 2007, 26, 7741–7748. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tang, X.; Guo, X.; Niikura, Y.; Kitagawa, K.; Cui, K.; Wong, S.T.; Fu, L.; Xu, B. Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Mol. Cell. 2011, 44, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Guo, X.; Qian, X.; Wang, H.; Yang, C.; Brinkman, K.L.; Serrano-Gonzalez, M.; Jope, R.S.; Zhou, B.; Engler, D.A.; et al. Activation of the ATM-Snail pathway promotes breast cancer metastasis. J. Mol. Cell Biol. 2012, 4, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158, Erratum in Proc. Natl. Acad. Sci. USA 2012, 109, 8352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, A.; Ralser, M. ATM is a redox sensor linking genome stability and carbon metabolism. Sci. Signal. 2011, 4, pe17. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Carballo, G.; Moreno, L.; Masiá, S.; Pérez, P.; Barettino, D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J. Biol. Chem. 2002, 277, 25297–25304. [Google Scholar] [CrossRef] [Green Version]
- Boehrs, J.K.; He, J.; Halaby, M.J.; Yang, D.Q. Constitutive expression and cytoplasmic compartmentalization of ATM protein in differentiated human neuron-like SH-SY5Y cells. J. Neurochem. 2007, 100, 337–345. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, H.; Yang, D.Q. Functional switching of ATM: Sensor of DNA damage in proliferating cells and mediator of Akt survival signal in post-mitotic human neuron-like cells. Chin. J. Cancer 2012, 31, 364–372. [Google Scholar] [CrossRef]
- Antonelli-Orlidge, A.; Saunders, K.B.; Smith, S.R.; D’Amore, P.A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc. Natl. Acad. Sci. USA 1989, 86, 4544–4548. [Google Scholar] [CrossRef] [Green Version]
- Fillinger, M.F.; Sampson, L.N.; Cronenwett, J.L.; Powell, R.J.; Wagner, R.J. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J. Surg. Res. 1997, 67, 169–178. [Google Scholar] [CrossRef]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Bourcier, T.; Bonnefoy, J.Y.; Pober, J.S.; Libby, P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1931–1936. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.K.; Rohovsky, S.A.; Beck, L.H.; Smith, S.R.; D’Amore, P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 1999, 84, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Zhang, G.; Chen, S.Y. Smooth Muscle Cell Proangiogenic Phenotype Induced by Cyclopentenyl Cytosine Promotes Endothelial Cell Proliferation and Migration. J. Biol. Chem. 2016, 291, 26913–26921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Gerthoffer, W.T.; Singer, C.A. Secretory Functions of Smooth Muscle: Cytokines and Growth Factors. Mol. Interv. 2002, 2, 447–456. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, P.A.; Smith, S.R. Growth factor effects on cells of the vascular wall: A survey. Growth Factors 1993, 8, 61–75. [Google Scholar] [CrossRef]




| Abbreviations | Names in Extenso | Phosphorylation Site (s) | References * |
|---|---|---|---|
| Akt/PKB | Akt/Protein Kinase B | Ser473 | 102–104 |
| ATF-2 | Activating Transcription Factor-2 | Thr69/Thr71 | 81 |
| ATM | Ataxia-Telangiectasia Mutated Ser/Thr kinase | Ser1981 | 82 |
| c-Fos | Protein of transcription factor AP1 | Thr232 | |
| c-Jun | Protein of transcription factor AP1 | Ser73 | 82 |
| CREB | cyclic AMP Response Element Binding protein | Ser133 | 77 |
| eIF-2α | eukaryotic translation Initiation Factor-2α | Ser51 | |
| HDAC-2 | Histone Deacetylase-2 | Ser394 | |
| HDAC-4 | Histone Deacetylase-4 | Ser632 | |
| IκBα | NF-κB Inhibitor α | Ser32 | 90 |
| MSK-1 | Mitogen- and Stress-activated protein Kinase | Ser376 | |
| NF-κB | Nuclear Factor-κB | Ser536 | 85 |
| SMAD-1 | Small Mother Against Decapentaplegic homolog-1 | Ser463/Ser465 | 78–80 |
| SMAD-2 | Small Mother Against Decapentaplegic homolog-2 | Ser245/Ser250/Ser255 | |
| SMAD-4 | Small Mother Against Decapentaplegic homolog-4 | Thr277 | |
| SMAD-5 | Small Mother Against Decapentaplegic homolog-5 | Ser463/Ser465 | |
| TAK-1 | TGF-β-Activated Kinase 1 | Ser412 | 76 |
| ZAP-70 | ZAP-70 Tyrosine protein kinase | Tyr292 |
| AGF | Trophic and Angiogenic Actions |
|---|---|
| IL-1α | |
| IL-2 | |
| IL-4 | |
| IL-6 | |
| IL-8 GRO-α/β/γ | |
| bFGF | |
| GM-CSF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Chiarini, A.; Wu, J.; Wei, Z.; Armato, U.; Dal Prà, I. Adult Human Vascular Smooth Muscle Cells on 3D Silk Fibroin Nonwovens Release Exosomes Enriched in Angiogenic and Growth-Promoting Factors. Polymers 2022, 14, 697. https://doi.org/10.3390/polym14040697
Hu P, Chiarini A, Wu J, Wei Z, Armato U, Dal Prà I. Adult Human Vascular Smooth Muscle Cells on 3D Silk Fibroin Nonwovens Release Exosomes Enriched in Angiogenic and Growth-Promoting Factors. Polymers. 2022; 14(4):697. https://doi.org/10.3390/polym14040697
Chicago/Turabian StyleHu, Peng, Anna Chiarini, Jun Wu, Zairong Wei, Ubaldo Armato, and Ilaria Dal Prà. 2022. "Adult Human Vascular Smooth Muscle Cells on 3D Silk Fibroin Nonwovens Release Exosomes Enriched in Angiogenic and Growth-Promoting Factors" Polymers 14, no. 4: 697. https://doi.org/10.3390/polym14040697
APA StyleHu, P., Chiarini, A., Wu, J., Wei, Z., Armato, U., & Dal Prà, I. (2022). Adult Human Vascular Smooth Muscle Cells on 3D Silk Fibroin Nonwovens Release Exosomes Enriched in Angiogenic and Growth-Promoting Factors. Polymers, 14(4), 697. https://doi.org/10.3390/polym14040697