Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects
Abstract
:1. Introduction
2. Materials and Methods
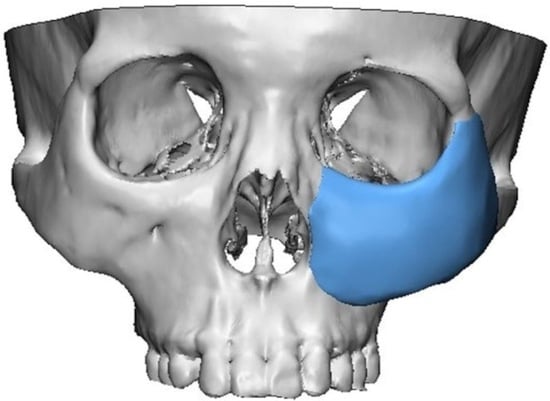
2.1. 3D Simulation and 3D Printing of Patient-Specific Implants
2.2. Surgical Procedure
2.3. Volume and Density Analysis Based on CT Data
2.4. Tensile Test of the Scaffold
3. Results
3.1. Case Presentation
3.1.1. Case 1
3.1.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chepeha, D.B.; Wang, S.J.; Marentette, L.J.; Bradford, C.R.; Boyd, C.M.; Prince, M.E.; Teknos, T.N. Restoration of the orbital aesthetic subunit in complex midface defects. Laryngoscope 2004, 114, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Liu, Y.; Gao, N.; Cai, J.; He, W.; Qiu, W. Reconstruction of Maxillary and Orbital Floor Defect With Free Fibula Flap and Whole Individualized Titanium Mesh Assisted by Computer Techniques. J. Oral Maxillofac. Surg. 2017, 75, 1791.e1. [Google Scholar] [CrossRef] [PubMed]
- Orringer, J.S.; Barcelona, V.; Buchman, S.R. Reasons for removal of rigid internal fixation devices in craniofacial surgery. J. Craniofac. Surg. 1998, 9, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Perrott, D.H.; Mahan, D.; Kearns, G. The removal of plates and screws after Le Fort I osteotomy. J. Oral Maxillofac. Surg. 1998, 56, 184–188. [Google Scholar] [CrossRef]
- Bell, R.B.; Kindsfater, C.S. The use of biodegradable plates and screws to stabilize facial fractures. J. Oral Maxillofac. Surg. 2006, 64, 31–39. [Google Scholar] [CrossRef]
- Mackool, R.; Yim, J.; McCarthy, J.G. Delayed degradation in a resorbable plating system. J. Craniofac. Surg. 2006, 17, 194–197, discussion 197–198. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schultze-Mosgau, S.; Schrell, U.; Wénzel, D.; Kessler, P. Biodegradable miniplates (LactoSorb): Long-term results in infant minipigs and clinical results. J. Craniofac. Surg. 2000, 11, 239–243, discussion 244–235. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ciocca, L.; Scotti, R.; Cipriani, R.; Marchetti, C. Surgical reconstruction of maxillary defects using a computer-assisted design/computer-assisted manufacturing-produced titanium mesh supporting a free flap. J. Craniomaxillofac. Surg. 2016, 44, 1320–1326. [Google Scholar] [CrossRef]
- Zhang, W.B.; Yu, Y.; Mao, C.; Wang, Y.; Guo, C.B.; Yu, G.Y.; Peng, X. Outcomes of Zygomatic Complex Reconstruction With Patient-Specific Titanium Mesh Using Computer-Assisted Techniques. J. Oral Maxillofac. Surg. 2019, 77, 1915–1927. [Google Scholar] [CrossRef]
- Lethaus, B.; Kessler, P.; Boeckman, R.; Poort, L.J.; Tolba, R. Reconstruction of a maxillary defect with a fibula graft and titanium mesh using CAD/CAM techniques. Head Face Med. 2010, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mir, S.M.; Aldulijan, I.; Mahajan, A.; Anwar, A.; Leon, C.H.; Terracciano, A.; Zhao, X.; Su, T.L.; Kalyon, D.M.; et al. Load-bearing biodegradable PCL-PGA-beta TCP scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 193–200. [Google Scholar] [CrossRef]
- Mkhabela, V.J.; Ray, S.S. Poly(epsilon-caprolactone) nanocomposite scaffolds for tissue engineering: A brief overview. J. Nanosci. Nanotechnol. 2014, 14, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Vizesi, F.; Michael, D.; Auld, J.; Langdown, A.; Oliver, R.; Yu, Y.; Irie, H.; Bruce, W. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials 2008, 29, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Vaněček, V.; Klíma, K.; Kohout, A.; Foltán, R.; Jiroušek, O.; Šedý, J.; Štulík, J.; Syková, E.; Jendelová, P. The combination of mesenchymal stem cells and a bone scaffold in the treatment of vertebral body defects. Eur. Spine J. 2013, 22, 2777–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.H.; Yoon, M.C.; Jeong, C.M.; Jang, J.; Jeong, S.I.; Cho, D.W.; Huh, J.B. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014, 9, 065006. [Google Scholar] [CrossRef]
- Shim, J.H.; Huh, J.B.; Park, J.Y.; Jeon, Y.C.; Kang, S.S.; Kim, J.Y.; Rhie, J.W.; Cho, D.W. Fabrication of blended polycaprolactone/poly(lactic-co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng. Part A 2013, 19, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Choi, D.; Shim, J.H.; Nam, W. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 2020, 10, 4979. [Google Scholar] [CrossRef]
- Mellor, L.F.; Nordberg, R.C.; Huebner, P.; Mohiti-Asli, M.; Taylor, M.A.; Efird, W.; Oxford, J.T.; Spang, J.T.; Shirwaiker, R.A.; Loboa, E.G. Investigation of multiphasic 3D-bioplotted scaffolds for site-specific chondrogenic and osteogenic differentiation of human adipose-derived stem cells for osteochondral tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2017–2030. [Google Scholar] [CrossRef] [Green Version]
- Khojasteh, A.; Behnia, H.; Hosseini, F.S.; Dehghan, M.M.; Abbasnia, P.; Abbas, F.M. The effect of PCL-TCP scaffold loaded with mesenchymal stem cells on vertical bone augmentation in dog mandible: A preliminary report. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 848–854. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Roelher, J.A.; Hench, L.L.; Maquet, V.; Jérǒme, R. A Composites Approach to Tissue Engineering. In Proceedings of the 26th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: B: Ceramic Engineering and Science Proceedings, Cocoa Beach, FL, USA, 13–18 January 2022; pp. 805–816. [Google Scholar] [CrossRef]
- Brown, J.S.; Shaw, R.J. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010, 11, 1001–1008. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Rai, B.; Sju, E.; Cheong, J.J.; Teoh, S.H. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: An in vitro and in vivo study. J. Biomed. Mater. Res. A 2008, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, É.; Magyar, L.; Bojtár, I. Material Properties of the Mandibular Trabecular Bone. J. Med. Eng. 2014, 2014, 470539. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, G.; Kim, C.; Lee, J.S.; Lee, I.G.; An, S.H.; Yun, W.S.; Kim, S.Y.; Shim, J.H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, e1800025. [Google Scholar] [CrossRef]
- Milne, N.; Fitton, L.; Kupczik, K.; Fagan, M.; O’Higgins, P. The role of the zygomaticomaxillary suture in modulating strain distribution within the skull of Macaca fascicularis. HOMO J. Comp. Hum. Biol. 2009, 281. [Google Scholar]
- Park, Y.J.; Cha, J.H.; Bang, S.I.; Kim, S.Y. Clinical Application of Three-Dimensionally Printed Biomaterial Polycaprolactone (PCL) in Augmentation Rhinoplasty. Aesthetic Plast. Surg. 2019, 43, 437–446. [Google Scholar] [CrossRef]
- Han, H.H.; Shim, J.H.; Lee, H.; Kim, B.Y.; Lee, J.S.; Jung, J.W.; Yun, W.S.; Baek, C.H.; Rhie, J.W.; Cho, D.W. Reconstruction of Complex Maxillary Defects Using Patient-specific 3D-printed Biodegradable Scaffolds. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1975. [Google Scholar] [CrossRef]
- Kim, S.Y. Application of the three-dimensionally printed biodegradable polycaprolactone (PCL) mesh in repair of orbital wall fractures. J. Craniomaxillofac. Surg. 2019, 47, 1065–1071. [Google Scholar] [CrossRef]
- Park, S.H.; Yun, B.G.; Won, J.Y.; Yun, W.S.; Shim, J.H.; Lim, M.H.; Kim, D.H.; Baek, S.A.; Alahmari, Y.D.; Jeun, J.H.; et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope 2017, 127, 1036–1043. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, I.H.; Yun, W.S.; Shim, J.H.; Choi, D.; Hwang, S.H.; Kim, S.W. Long-term efficacy and safety of 3D printed implant in patients with nasal septal deformities. Eur. Arch. Oto-Rhino-Laryngol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sivaloganathan, S.; Amr, R.; Shrivastava, R.; Relwani, J. The Risotto sign - a severe inflammatory bursitis with rice body formation, complicating a rotator cuff repair with a bioabsorbable suture anchor. JRSM Open 2015, 6, 2054270414562986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrokalos, D.S.; Paessler, H.H. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy 2008, 24, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.M.; Mafong, E.A.; Kauvar, A.N.; Geronemus, R.G. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol. Surg. 2002, 28, 491–494. [Google Scholar] [CrossRef]
- Skrzypek, E.; Górnicka, B.; Skrzypek, D.M.; Krzysztof, M.R. Granuloma as a complication of polycaprolactone-based dermal filler injection: Ultrasound and histopathology studies. J. Cosmet. Laser Ther. 2019, 21, 65–68. [Google Scholar] [CrossRef]
- Chiang, C.H.; Peng, J.H.; Peng, H.P. Filler-induced granuloma from polycaprolactone-based collagen stimulator injection in the tear trough area: A case report. J. Cosmet. Dermatol. 2021, 20, 1529–1531. [Google Scholar] [CrossRef]
- Yun, S.; Choi, D.; Choi, D.J.; Jin, S.; Yun, W.S.; Huh, J.B.; Shim, J.H. Bone Fracture-Treatment Method: Fixing 3D-Printed Polycaprolactone Scaffolds with Hydrogel Type Bone-Derived Extracellular Matrix and β-Tricalcium Phosphate as an Osteogenic Promoter. Int. J. Mol. Sci. 2021, 22, 9084. [Google Scholar] [CrossRef]
- Bae, E.B.; Park, K.H.; Shim, J.H.; Chung, H.Y.; Choi, J.W.; Lee, J.J.; Kim, C.H.; Jeon, H.J.; Kang, S.S.; Huh, J.B. Efficacy of rhBMP-2 Loaded PCL/β-TCP/bdECM Scaffold Fabricated by 3D Printing Technology on Bone Regeneration. Biomed. Res. Int. 2018, 2018, 2876135. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, J.S.; Oh, E.J.; Kim, T.J.; Kim, H.M.; Shim, J.H.; Yoon, W.S.; Huh, J.B.; Moon, S.H.; Kang, S.S.; et al. Effects of three-dimensionally printed polycaprolactone/β-tricalcium phosphate scaffold on osteogenic differentiation of adipose tissue- and bone marrow-derived stem cells. Arch. Craniofac. Surg. 2018, 19, 181–189. [Google Scholar] [CrossRef]
- Shin, Y.M.; Park, J.-S.; Jeong, S.I.; An, S.-J.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, C.-Y. Promotion of human mesenchymal stem cell differentiation on bioresorbable polycaprolactone/biphasic calcium phosphate composite scaffolds for bone tissue engineering. Biotechnol. Bioprocess Eng. 2014, 19, 341–349. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wu, P.; Gao, C.; Yang, Y.; Guo, W.; Yang, W.; Shuai, C. A Multimaterial Scaffold With Tunable Properties: Toward Bone Tissue Repair. Adv. Sci. 2018, 5, 1700817. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G. Functionally graded PCL/β-TCP biocomposites in a multilayered structure for bone tissue regeneration. Appl. Phys. A 2012, 108, 949–959. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J. Mater. Sci. Mater. Med. 2012, 23, 2217–2226. [Google Scholar] [CrossRef]
- Stal, S.; Hollier, L. The use of resorbable spacers for nasal spreader grafts. Plast. Reconstr. Surg. 2000, 106, 922–928, discussion 929–931. [Google Scholar] [CrossRef]
- Schuckert, K.H.; Jopp, S.; Teoh, S.H. Mandibular defect reconstruction using three-dimensional polycaprolactone scaffold in combination with platelet-rich plasma and recombinant human bone morphogenetic protein-2: De novo synthesis of bone in a single case. Tissue Eng. Part A 2009, 15, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Matinlinna, J.P.; Tsoi, J.K.H.; Liu, W.; Cui, X.; Lu, W.W.; Pan, H. Recent developments in biomaterials for long-bone segmental defect reconstruction: A narrative overview. J. Orthop. Translat. 2020, 22, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tong, W.; Luria, J.S.; Wang, Z.; Nussenbaum, B.; Krebsbach, P.H. Effects of bone morphogenetic protein-2 on proliferation and angiogenesis in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2010, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]








| Sex | Age | Cause of Defect | Location | Type of Defect | Onset of Reconstruction | Postoperative Complication | Underlying Disease | |
|---|---|---|---|---|---|---|---|---|
| Patient #1 | F | 21 | Intraosseous hemangioma | Rt. | N.A. | 24-month delayed | None | None |
| Patient #2 | M | 19 | Romberg disease | Rt. | N.A | Delayed | None | None |
| Patient #3 | M | 51 | Intraosseous hemangioma | Lt. | V | Immediate | None | None |
| Patient #4 | F | 50 | Traumatic facial deformity | Lt. | N.A | 60-month delayed | None | None |
| Patient #5 | M | 21 | Fibrous dysplasia | Lt. | IIIb | Immediate | None | None |
| Patient #6 | F | 43 | Radiation necrosis following nasal cavity cancer ablation | Lt. | IIIb | Immediate | Wound dehiscence due to delayed wound healing | Diabetes |
| Patient #7 | F | 44 | Radiation necrosis following maxillary sinus cancer ablation | Lt. | IIIb | Immediate | None | Hypertension |
| Patient #8 | M | 42 | Maxillary sinus cancer | Rt. | V | Immediate | None | None |
| Reconstructive Option | Incisional Approach | Application of Bone Regeneration Material | Implant Fixation | Revisional Operation | |
|---|---|---|---|---|---|
| Patient #1 | Fat graft | Gingivobuccal and transconjunctival | None | HA-PLLA resorbable plate and screws | Secondary fat graft |
| Patient #2 | Fat graft | Gingivobuccal and transconjunctival | Resorbable calcium phosphate bone substitute | Titanium miniplate and screws | Secondary fat graft |
| Patient #3 | Fat graft | Gingivobuccal and transconjunctival | DBM | Titanium miniplate and screws | Secondary fat graft |
| Patient #4 | Fat graft | Gingivobuccal and transconjunctival | None | Titanium miniplate and screws | Secondary fat graft |
| Patient #5 | Iliac bone graft | Gingivobuccal and transconjunctival | DBM | Wire steel | None |
| Patient #6 | RFFF, Iliac bone graft | Weber-Ferguson approach | None | Titanium miniplate and screws | Local wound coverage |
| Patient #7 | ALT FF, RFFF | Lateral rhinotomy and subcillary approach | None | Titanium miniplate and screws | None |
| Patient #8 | None | Lateral rhinotomy and subcillary approach | None | Wire steel | None |
| Preoperatively Planned Implant Volume (mm3) | Postoperative Actual Implant Volume (mm3) | Conforming Volume after Superimposition (mm3) | Volume Conformity (%) | Postoperative Newly Generated Bone Volume (mm3) | Bone Volume Fraction (%) | Postoperative Mean Tissue Density (HU) | |
|---|---|---|---|---|---|---|---|
| Patient #1 | 11.82 | 10.55 | 9.62 | 81.39 | 1.25 | 11.87 | 165.55 |
| Patient #2 | 8.76 | 8.42 | 7.51 | 85.77 | 3.15 | 37.41 | 184.22 |
| Patient #3 | 3.72 | 3.22 | 2.64 | 70.89 | 0.25 | 7.81 | 223.00 |
| Patient #4 | 2.16 | 1.84 | 1.66 | 76.76 | 1.22 | 66.21 | 291.74 |
| Patient #5 | 30.37 | 28.22 | 26.22 | 86.31 | 7.15 | 25.34 | 184.55 |
| Patient #6 | 15.88 | 13.51 | 11.53 | 72.59 | 2.13 | 15.73 | 168.44 |
| Patient #7 | 2.74 | 2.49 | 2.16 | 79.05 | 0.22 | 8.80 | 151.48 |
| Patient #8 | 15.09 | 13.42 | 12.82 | 84.96 | 1.82 | 13.54 | 182.51 |
| Scaffold Dimension (mm) | Porosity (%) | Young’s Modulus | Number of Sample |
|---|---|---|---|
| 10 × 40 × 1 | 50 | 162.7 ± 12. 8 MPa | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. https://doi.org/10.3390/polym14040740
Jeong W-S, Kim Y-C, Min J-C, Park H-J, Lee E-J, Shim J-H, Choi J-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers. 2022; 14(4):740. https://doi.org/10.3390/polym14040740
Chicago/Turabian StyleJeong, Woo-Shik, Young-Chul Kim, Jae-Cheong Min, Ho-Jin Park, Eun-Ju Lee, Jin-Hyung Shim, and Jong-Woo Choi. 2022. "Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects" Polymers 14, no. 4: 740. https://doi.org/10.3390/polym14040740
APA StyleJeong, W. -S., Kim, Y. -C., Min, J. -C., Park, H. -J., Lee, E. -J., Shim, J. -H., & Choi, J. -W. (2022). Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers, 14(4), 740. https://doi.org/10.3390/polym14040740