Static Permittivity and Electro-Optical Properties of Bi-Component Orthoconic Antiferroelectric Liquid Crystalline Mixtures Targeted for Polymer Stabilized Sensing Systems
Abstract
:1. Introduction
2. Materials and Methods
 (S)-4′-(1-methyloctyloxycarbonyl)biphenyl-4-yl4-[3-(2,2,3,3,4,4,4-heptafluorobutoxy)prop-1-oxy]-2-fluorobenzoate (Compound A) [63]
(S)-4′-(1-methyloctyloxycarbonyl)biphenyl-4-yl4-[3-(2,2,3,3,4,4,4-heptafluorobutoxy)prop-1-oxy]-2-fluorobenzoate (Compound A) [63] (S)-4′-(1-methylpentyloxycarbonyl)biphenyl-4-yl4-[5-(2,2,3,3,4,4,4-heptafluorobutoxy) pentyl-1-oxy]-2-fluorobenzoate (Compound B) [63]
(S)-4′-(1-methylpentyloxycarbonyl)biphenyl-4-yl4-[5-(2,2,3,3,4,4,4-heptafluorobutoxy) pentyl-1-oxy]-2-fluorobenzoate (Compound B) [63] (S)-4′-(1-methylcyclohexyloxycarbonyl)biphenyl-4-yl4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl-1-oxy]benzoate (Compound C) [63]
(S)-4′-(1-methylcyclohexyloxycarbonyl)biphenyl-4-yl4-[7-(2,2,3,3,4,4,4-heptafluorobutoxy)heptyl-1-oxy]benzoate (Compound C) [63]3. Results
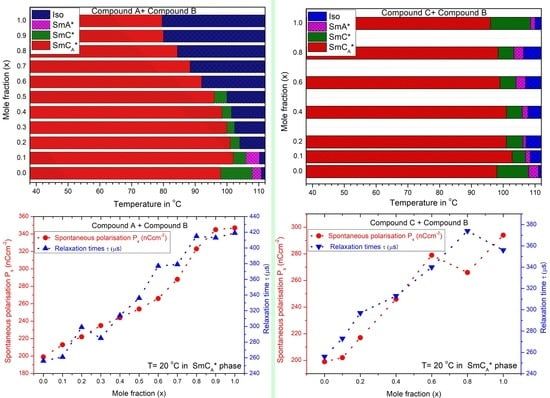
3.1. Mesomorphic Behavior
3.2. Static Permittivity and Dielectric Anisotropy
3.3. Helical Pitch
3.4. Spontaneous Polarization
3.5. Relaxation Time
3.6. Effective Torsional Bulk Viscosity
3.7. Anchoring Energy Coefficients
3.7.1. Dispersion Anchoring Energy Coefficient
3.7.2. Polarization Anchoring Energy Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinitzer, F. Contributions to the knowledge of cholesterol. Liq. Cryst. 1989, 5, 7–18. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Scalia, G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. [Google Scholar] [CrossRef]
- Meyer, R.B.; Liebert, L.; Strzelecki, L.; Keller, P. Ferroelectric liquid crystals. J. Phys. Lett. 1975, 36, 69–71. [Google Scholar] [CrossRef]
- Clark, N.A.; Lagerwall, S.T. Submicrosecond bistable electro-optic switching in liquid crystals. Appl. Phys. Lett. 1980, 36, 899–901. [Google Scholar] [CrossRef]
- Chandani, A.D.L.; Hagiwara, T.; Suzuki, Y.; Ouchi, Y.; Takezoe, H.; Fukuda, A. Tristable Switching in Surface Stabilized Ferroelectric Liquid Crystals with a Large Spontaneous Polarization. Jpn. J. Appl. Phys. 1988, 27, L729–L732. [Google Scholar] [CrossRef]
- Chandani, A.D.L.; Gorecka, E.; Ouchi, Y.; Takezoe, H.; Fukuda, A. Antiferroelectric Chiral Smectic Phases Responsible for the Trislable Switching in {MHPOBC}. Jpn. J. Appl. Phys. 1989, 28, L1265–L1268. [Google Scholar] [CrossRef]
- Garoff, S.; Meyer, R.B. Electroclinic Effect at the A–C Phase Change in a Chiral Smectic Liquid Crystal. Phys. Rev. Lett. 1977, 38, 848–851. [Google Scholar] [CrossRef]
- Nishiyama, S.; Ouchi, Y.; Takezoe, H.; Fukuda, A. Giant Electroclinic Effect in Chiral Smectic a Phase of Ferroelectric Liquid Crystals. Jpn. J. Appl. Phys. 1987, 26, L1787–L1789. [Google Scholar] [CrossRef]
- Davey, A.B.; Crossland, W.A. Potential and limitations of the electroclinic effect in device applications. Ferroelectrics 1991, 114, 101–112. [Google Scholar] [CrossRef]
- Malik, A.; Choudhary, A.; Silotia, P.; Biradar, A.M.; Singh, V.K.; Kumar, N. Effect of graphene oxide nanomaterial in electroclinic liquid crystals. J. Appl. Phys. 2010, 108, 124110–124116. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Choudhary, A.; Kaur, S.; Biradar, A.; Haase, W. Reminiscence Shown by de Vries Electroclinic Liquid Crystal Material. Jpn. J. Appl. Phys. 2007, 46, L559. [Google Scholar] [CrossRef]
- Singh, G.; Prakash, G.V.; Choudhary, A.; Biradar, A.M. Electro-optic studies in conventional and pure/ethanol mixed de Vries ferroelectric liquid crystals. Liq. Cryst. 2012, 39, 185–190. [Google Scholar] [CrossRef]
- Choudhary, A.; Kaur, S.; Singh, G.; Prakash, J.; Thakur, A.K.; Biradar, A.M. Memory effect in Sm C* phase of electroclinic liquid crystals. J. Appl. Phys. 2007, 101, 74112. [Google Scholar] [CrossRef]
- Glogarová, M.; Pavel, J.; Bawa, S.S.; Demus, D.; Diele, S.; Pelzl, G. Macroscopic properties of mixed chiral smectic liquid crystals. Liq. Cryst. 1988, 3, 353–361. [Google Scholar] [CrossRef]
- Dozov, I. 26.1: Invited Paper: Bistable Liquid Crystal Technologies. SID Symp. Dig. Tech. Pap. 2003, 34, 946–949. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Chigrinov, V.G. Bistable and multistable states in ferroelectric liquid crystals. Crystallogr. Rep. 2006, 51, 1030–1040. [Google Scholar] [CrossRef]
- Talarico, M.; Golemme, A. Bistable Switching in Photorefractive Surface Stabilized Ferroelectric Liquid Crystals. Mol. Cryst. Liq. Cryst. 2007, 465, 341–358. [Google Scholar] [CrossRef]
- Sutkowski, M.; Grudniewski, T.; Piecek, W.; Morawiak, P.; Parka, J.; Nowinowski-Kruszelnicki, E.; Raszewski, Z. Holographic Recordings Using Bistable SmC * Structures. Mol. Cryst. Liq. Cryst. 2009, 502, 195–206. [Google Scholar] [CrossRef]
- Sutkowski, M.; Piecek, W.; Grudniewski, T.; Parka, J.; Nowinowski-Kruszelnicki, E. Light driven optical switching of the surface stabilized antiferroelectric liquid crystals. Opt. Lasers Eng. 2011, 49, 1330–1334. [Google Scholar] [CrossRef]
- O’Callaghan, M.J.; Wand, M.; Walker, C.; Thurmes, W.; More, K. High-Tilt, High-PS, de Vries FLCs for Analog Electro-Optic Phase Modulation. Ferroelectrics 2006, 343, 201–207. [Google Scholar] [CrossRef]
- Petrenko, A.; Goodby, J.W. V-Shaped switching and interlayer interactions in ferroelectric liquid crystals. J. Mater. Chem. 2007, 17, 766–782. [Google Scholar] [CrossRef]
- Hammarquist, A.; D’Havé, K.; Matuszczyk, M.; Clark, N.A.; MacLennan, J.E.; Rudquist, P. V-shaped switching ferroelectric liquid crystal structure stabilized by dielectric surface layers. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2008, 77, 031707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urruchi, V.; Gaona, N.; Sánchez-Pena, J.M. Evaluation of the Phase Modulation During V-Shaped Switching in a Smectic Liquid Crystal Device. Mol. Cryst. Liq. Cryst. 2008, 494, 272–281. [Google Scholar] [CrossRef]
- Lagerwall, S.T. Ferroelectric and antiferroelectric liquid crystals. Ferroelectrics 2004, 301, 15–45. [Google Scholar] [CrossRef]
- Bos, P.; Bhowmik, A. Liquid-Crystal Technology Advances toward Future True 3-D Flat-Panel Displays. Inf. Disp. 2011, 27, 6–10. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef]
- Rudquist, P. Orthoconic antiferroelectric liquid crystals. Liq. Cryst. 2013, 40, 1678–1697. [Google Scholar] [CrossRef]
- D’havé, K.; Rudquist, P.; Lagerwall, S.T.; Pauwels, H.; Drzewinski, W.; Dabrowski, R. Solution of the dark state problem in antiferroelectric liquid crystal displays. Appl. Phys. Lett. 2000, 76, 3528–3530. [Google Scholar] [CrossRef]
- Johno, M.; Itoh, K.; Lee, J.; Ouchi, Y.; Takezoe, H.; Fukuda, A.; Kitazume, T. Temporal and Spatial Behavior of the Field-Induced Transition between Antiferroelectric and Ferroelectric Phases in Chiral Smectics. Jpn. J. Appl. Phys. 1990, 29, L107–L110. [Google Scholar] [CrossRef]
- Beccherelli, R.; Elston, S.J. Evaluation of optical anisotropy in the pretransitional regime in antiferroelectric liquid crystals. Liq. Cryst. 1998, 25, 573–577. [Google Scholar] [CrossRef]
- Pandey, M.; Verma, R.; Dhar, R. Dielectric and Electro-Optical Properties of Antiferroelectric Liquid Crystalline Materials. Isr. J. Chem. 2012, 52, 895. [Google Scholar] [CrossRef]
- Piecek, W.; Dbrowski, R.; Morawiak, P.; Urowska, M.; Jaroszewicz, L. The orthoconic antiferroelectric smectic liquid crystals and their engineering by doping with homo- and heterostructural compounds. Phase Transit. 2012, 85, 910–929. [Google Scholar] [CrossRef]
- D’Havé, K.; Dahlgren, A.; Rudquist, P.; Lagerwall, J.P.F.; Andersson, G.; Matuszczyk, M.; Lagerwall, S.T.; Dabrowski, R.; Drzewinski, W. Antiferroelectric liquid crystals with 45° tilt—A new class of promising electro-optic materials. Ferroelectrics 2000, 244, 115–128. [Google Scholar] [CrossRef]
- Lagerwall, S.T.; Dahlgren, A.; Jägemalm, P.; Rudquist, P.; D’havé, K.; Pauwels, H.; Dabrowski, R.; Drzewinski, W. Unique Electro-Optical Properties of Liquid Crystals Designed for Molecular Optics. Adv. Funct. Mater. 2001, 11, 87–94. [Google Scholar] [CrossRef]
- Piecek, W.; Perkowski, P.; Raszewski, Z.; Morawiak, P.; Żurowska, M.; Dąbrowski, R.; Czupryński, K. Long Pitch Orthoconic Antiferroelectric Binary Mixture for Display Applications. Mol. Cryst. Liq. Cryst. 2010, 525, 140–152. [Google Scholar] [CrossRef]
- Pandey, M.B.; Dabrowski, R.; Dhar, R. Investigation of relaxation processes in anticlinic smectic C* (Sm CA*) phase of Liquid Crystals by dielectric spectroscopy. Phys. B Condens. Matter 2007, 387, 25–31. [Google Scholar] [CrossRef]
- Engström, D.; Rudquist, P.; Bengtsson, J.; D’havé, K.; Galt, S. Three-level phase modulator based on orthoconic antiferroelectric liquid crystals. Opt. Lett. 2006, 31, 3158–3160. [Google Scholar] [CrossRef]
- Engström, D.; O’Callaghan, M.J.; Walker, C.; Handschy, M.A. Fast beam steering with a ferroelectric-liquid-crystal optical phased array. Appl. Opt. 2009, 48, 1721–1726. [Google Scholar] [CrossRef]
- Rudquist, P.; Elfström, D.; Lagerwall, S.T.; Dabrowski, R. Polymer-Stabilized Orthoconic Antiferroelectric Liquid Crystals. Ferroelectrics 2006, 344, 177–188. [Google Scholar] [CrossRef]
- Czerwiński, M.; Urbańska, M.; Bennis, N.; Rudquist, P. Influence of the type of phase sequence and polymer-stabilization on the physicochemical and electro-optical properties of novel high-tilt antiferroelectric liquid crystalline materials. J. Mol. Liq. 2019, 288, 111057. [Google Scholar] [CrossRef]
- Czerwiński, M.; Blas, M.; Bennis, N.; Herman, J.; Dmochowska, E.; Otón, J. Polymer stabilized highly tilted antiferroelectric liquid crystals—The influence of monomer structure and phase sequence of base mixtures. J. Mol. Liq. 2020, 327, 114869. [Google Scholar] [CrossRef]
- Engström, D.; Rudquist, P.; Bengtsson, J.; D’havé, K.; Galt, S. Near-lossless continuous phase modulation using the analog switching mode (V-shaped switching) in ferroelectric liquid crystals. Appl. Opt. 2006, 45, 5258–5269. [Google Scholar] [CrossRef]
- Seomun, S.S.; Takanishi, Y.; Ishikawa, K.; Takezoe, H.; Fukuda, A. Evolution of switching characteristics from tristable to V-shaped in an apparently antiferroelectric liquid crystal. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 1997, 36, 3586–3590. [Google Scholar] [CrossRef]
- Seomun, S. Bulk optical properties in binary mixtures of antiferroelectric liquid crystal compounds showing V-shaped switching. Liq. Cryst. 1999, 26, 151–161. [Google Scholar] [CrossRef]
- Pozhidaev, E.P.; Vashchenko, V.V.; Mikhailenko, V.V.; Krivoshey, A.I.; Barbashov, V.A.; Shi, L.; Srivastava, A.K.; Chigrinov, V.G.; Kwok, H.S. Ultrashort helix pitch antiferroelectric liquid crystals based on chiral esters of terphenyldicarboxylic acid. J. Mater. Chem. C 2016, 4, 10339–10346. [Google Scholar] [CrossRef]
- Brodzeli, Z.; Silvestri, L.; Michie, A.; Guo, Q.; Pozhidaev, E.; Chigrinov, V.G.; Ladouceur, F. Sensors at Your Fibre Tips: A Novel Liquid Crystal-Based Photonic Transducer for Sensing Systems. J. Light. Technol. 2013, 31, 2940–2946. [Google Scholar] [CrossRef]
- Brodzeli, Z.; Silvestri, L.; Michie, A.; Guo, Q.; Pozhidaev, E.P.; Chigrinov, V.; Ladouceur, F. Reflective mode of deformed-helix ferroelectric liquid crystal cells for sensing applications. Liq. Cryst. 2013, 40, 1427–1435. [Google Scholar] [CrossRef]
- Firth, J.; Ladouceur, F.; Brodzeli, Z.; Wyres, M.; Silvestri, L. A novel optical telemetry system applied to flowmeter networks. Flow Meas. Instrum. 2016, 48, 15–19. [Google Scholar] [CrossRef]
- Firth, J.; Ladouceur, F.; Brodzeli, Z.; Bruin, C.; Wang, H.; Silvestri, L. Liquid Crystal based optical telemetry applied to 4–20mA current loop networks. Sens. Actuators A Phys. 2017, 260, 124–130. [Google Scholar] [CrossRef]
- Firth, J.; Brodzeli, Z.; Ciobotaru, M.; Phung, T.; Brodzeli, Z.; Silvestri, L. Accurate optical measurement of high voltage waveform using novel optical liquid crystal based sensor. Sens. Actuators A Phys. 2017, 268. [Google Scholar] [CrossRef]
- Al Abed, A.; Srinivas, H.; Firth, J.; Ladouceur, F.; Lovell, N.H.; Silvestri, L. A biopotential optrode array: Operation principles and simulations. Sci. Rep. 2018, 8, 2690. [Google Scholar] [CrossRef] [PubMed]
- Chełstowska, A.; Czerwiński, M.; Tykarska, M.; Bennis, N. The influence of antiferroelectric compounds on helical pitch of orthoconic W-1000 mixture. Liq. Cryst. 2014, 41, 812–820. [Google Scholar] [CrossRef] [Green Version]
- Inui, S.; Iimura, N.; Suzuki, T.; Iwane, H.; Miyachi, K.; Takanishi, Y.; Fukuda, A. Thresholdless antiferroelectricity in liquid crystals and its application to displays. J. Mater. Chem. 1996, 6, 671–673. [Google Scholar] [CrossRef]
- Tykarska, M.; Skrzypek, K.; Ścibior, E.; Samsel, A. Helical Pitch in Bicomponent Mixtures with Induced Antiferroelectric Phase. Mol. Cryst. Liq. Cryst. 2006, 449, 71–77. [Google Scholar] [CrossRef]
- Fitas, J.; Marzec, M.; Kurp, K.; Żurowska, M.; Tykarska, M.; Bubnov, A. Electro-optic and dielectric properties of new binary ferroelectric and antiferroelectric liquid crystalline mixtures. Liq. Cryst. 2017, 44, 1468–1476. [Google Scholar] [CrossRef]
- Bubnov, A.; Vacek, C.; Czerwiński, M.; Vojtylová, T.; Piecek, W.; Hamplová, V. Design of polar self-assembling lactic acid derivatives possessing submicrometre helical pitch. Beilstein J. Nanotechnol. 2018, 9, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Kurp, K.; Czerwiński, M.; Tykarska, M.; Salamon, P.; Bubnov, A. Design of functional multicomponent liquid crystalline mixtures with nano-scale pitch fulfilling deformed helix ferroelectric mode demands. J. Mol. Liq. 2019, 290, 111329. [Google Scholar] [CrossRef]
- Piecek, W.; Bubnov, A.; Perkowski, P.; Morawiak, P.; Ogrodnik, K.; Rejmer, W.; Żurowska, M.; Hamplová, V.; Kašpar, M. An effect of structurally non-compatible additive on the properties of a long-pitch orthoconic antiferroelectric mixture. Phase Transit. 2010, 83, 551–563. [Google Scholar] [CrossRef]
- Żurowska, M.; Dziaduszek, J.; Szala, M.; Morawiak, P.; Bubnov, A. Effect of lateral fluorine substitution far from the chiral center on mesomorphic behaviour of highly titled antiferroelectric (S) and (R) enantiomers. J. Mol. Liq. 2017, 267, 504–510. [Google Scholar] [CrossRef]
- Novotná, V.; Hamplová, V.; Kašpar, M.; Glogarová, M.; Bubnov, A.; Lhotáková, Y. Phase Diagrams of Binary Mixtures of Antiferroelectric and Ferroelectric Compounds with Lactate Units in the Mesogenic Core. Ferroelectrics 2004, 309, 103–109. [Google Scholar] [CrossRef]
- Fitas, J.; Marzec, M.; Szymkowiak, M.; Jaworska-Gołąb, T.; Deptuch, A.; Tykarska, M.; Kurp, K.; Żurowska, M.; Bubnov, A. Mesomorphic, electro-optic and structural properties of binary liquid crystalline mixtures with ferroelectric and antiferroelectric liquid crystalline behaviour. Phase Transit. 2018, 91, 1017–1026. [Google Scholar] [CrossRef]
- Vojtylová-Jurkovičová, T.; Vaňkátová, P.; Urbańska, M.; Hamplová, V.; Sýkora, D.; Bubnov, A. Effective control of optical purity by chiral HPLC separation for ester-based liquid crystalline materials forming anticlinic smectic phases. Liq. Cryst. 2021, 48, 43–53. [Google Scholar] [CrossRef]
- Milewska, K.; DrzewiDski, W.; CzerwiDski, M.; Dabrowski, R. Design, synthesis and mesomorphic properties of chiral benzoates and fluorobenzoates with direct SmCA*-Iso phase transition. Liq. Cryst. 2015, 42, 1601–1611. [Google Scholar] [CrossRef]
- Milewska, K.; Drzewiński, W.; Czerwiński, M.; Dąbrowski, R.; Piecek, W. Highly tilted liquid crystalline materials possessing a direct phase transition from antiferroelectric to isotropic phase. Mater. Chem. Phys. 2016, 171, 33–38. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Garbat, K.; Filipowicz, M.; Tykarska, M.; Rejmer, W.; Czupryński, K.; Spadło, A.; Bennis, N.; et al. Influence of alkoxy chain length and fluorosubstitution on mesogenic and spectral properties of high tilted antiferroelectric esters. J. Mater. Chem. 2011, 21, 2144–2153. [Google Scholar] [CrossRef]
- Tykarska, M.; Czerwiński, M.; Żurowska, M.; Czerwí, M.; Urbańska, M. The temperature and concentration dependence of helical pitch in the mixtures of antiferroelectric compounds with the opposite helical twist sense. Liq. Cryst. 2011, 38, 561–566. [Google Scholar] [CrossRef]
- Pramanik, A.; Das, M.K.; Das, B.; Żurowska, M.; Dąbrowski, R. Electro-optical properties of a new series of fluorinated antiferroelectric orthoconic liquid crystalline esters. Liq. Cryst. 2015, 42, 412–421. [Google Scholar] [CrossRef]
- Barman, B.; Das, B.; Das, M.K.; Hamplová, V.; Bubnov, A. Effect of molecular structure on dielectric and electro-optic properties of chiral liquid crystals based on lactic acid derivatives. J. Mol. Liq. 2019, 283, 472–481. [Google Scholar] [CrossRef]
- Nepal, S.; Mondal, S.; Sinha, A.; Das, B.; Das, M.K.; Dmochowska, E.; Herman, J.; Czerwiński, M. Fast switching behaviour and dielectric parameters of two chiral ferroelectric mesogens. Liq. Cryst. 2020, 47, 1464–1472. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, M.K.; Bubnov, A.; Weissflog, W.; Węgłowska, D.; Dabrowski, R. Induced frustrated twist grain boundary liquid crystalline phases in binary mixtures of achiral hockey stick-shaped and chiral rod-like materials. J. Mater. Chem. C 2019, 7, 10530–10543. [Google Scholar] [CrossRef]
- Nepal, S.; Das, B.; Das, M.K.; Sarkar, M.D.; Dabrowski, R. Dielectric spectroscopy and electrical conductivity measurements of a series of orthoconic antiferroelectric liquid crystalline esters. Ferroelectrics 2021, 570, 100–114. [Google Scholar] [CrossRef]
- Nepal, S.; Das, B.; Das, M.K.; Strójwąs, K.; Urbańska, M. Dielectric spectroscopy and electrical conductivity measurements on high-tilted antiferroelectric materials. Phase Transit. 2020, 93, 909–923. [Google Scholar] [CrossRef]
- Malik, P.; Raina, K.; Bubnov, A.; Chaudhary, A.; Singh, R. Electro-optic switching and dielectric spectroscopy studies of ferroelectric liquid crystals with low and high spontaneous polarization. Thin Solid Films 2010, 519, 1052–1055. [Google Scholar] [CrossRef]
- Malik, P.; Raina, K.K.; Bubnov, A.; Prakash, C. Dielectric spectroscopy of a high-polarization ferroelectric liquid crystal. Phase Transit. 2006, 79, 889–898. [Google Scholar] [CrossRef]
- Rychetský, I.; Glogarová, M.; Bubnov, A.M. Dynamic dielectric response of SmC* thin layer in planar geometry-thickness mode relaxation. Ferroelectrics 1998, 212, 21–28. [Google Scholar] [CrossRef]
- Gouda, F.; Skarp, K.; Lagerwall, S.T. Dielectric studies of the soft mode and Goldstone mode in ferroelectric liquid crystals. Ferroelectrics 1991, 113, 165–206. [Google Scholar] [CrossRef]
- Pramanik, A.; Das, M.K.; Das, B.; Dąbrowski, R. Preparation and Study of the Electro-optical Properties of Binary Mixtures of Orthoconic Anti-ferroelectric Esters and Achiral Phenyl Pyrimidine Liquid Crystal. Soft Mater. 2015, 13, 201–209. [Google Scholar] [CrossRef]
- Allagulov, A.I.; Pikin, S.A.; Chigrinov, V.G. Bistable and monostable polarized states of a liquid-crystalline ferroelectric in an electric field. Liq. Cryst. 1989, 5, 1099–1105. [Google Scholar] [CrossRef]
- Misra, A.K.; Srivastava, A.K.; Shukla, J.P.; Manohar, R. Dielectric and electro-optical parameters of two ferroelectric liquid crystals: A comparative study. Phys. Scr. 2008, 78, 065602. [Google Scholar]
- Manohar, R.; Yadav, S.P.; Pandey, K.K.; Srivastava, A.K.; Misra, A.K. Comparative study of dielectric and electro-optical properties of pure and polymer ferroelectric liquid crystal composites. J. Polym. Res. 2011, 18, 435–441. [Google Scholar] [CrossRef]
























| Mole Fraction (x) | Ps (nC·cm−2) | τ (µs) | ƞ (mPa.s) | Wd (J·m−2) | Wp (J·m−2) |
|---|---|---|---|---|---|
| 0.0 | 199 | 256 | 2600 | 0.020 | 0.016 |
| 0.1 | 213 | 261 | 3568 | 0.021 | 0.017 |
| 0.2 | 222 | 299 | 3567 | 0.022 | 0.017 |
| 0.3 | 235 | 285 | 4548 | 0.024 | 0.019 |
| 0.4 | 244 | 314 | 5400 | 0.024 | 0.020 |
| 0.5 | 254 | 336 | 5577 | 0.025 | 0.020 |
| 0.6 | 266 | 377 | 7186 | 0.025 | 0.021 |
| 0.7 | 288 | 379 | 8090 | 0.028 | 0.024 |
| 0.8 | 323 | 415 | 8945 | 0.031 | 0.026 |
| 0.9 | 345 | 413 | 10102 | 0.034 | 0.027 |
| 1.0 | 347 | 419 | 10554 | 0.034 | 0.028 |
| Mole Fraction (x) | Ps (nC.cm−2) | τ (µs) | ƞ (mPa.s) | Wd (J.m−2) | Wp (J.m−2) |
|---|---|---|---|---|---|
| 0.0 | 199 | 256 | 2600 | 0.019 | 0.016 |
| 0.1 | 202 | 273 | 5470 | 0.020 | 0.018 |
| 0.2 | 217 | 297 | 5653 | 0.021 | 0.018 |
| 0.4 | 246 | 313 | 6573 | 0.024 | 0.020 |
| 0.6 | 279 | 340 | 7529 | 0.026 | 0.021 |
| 0.8 | 266 | 374 | 8400 | 0.025 | 0.020 |
| 1.0 | 294 | 356 | 6639 | 0.027 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepal, S.; Das, B.; Das, M.K.; Sarkar, M.D.; Urbańska, M.; Czerwiński, M. Static Permittivity and Electro-Optical Properties of Bi-Component Orthoconic Antiferroelectric Liquid Crystalline Mixtures Targeted for Polymer Stabilized Sensing Systems. Polymers 2022, 14, 956. https://doi.org/10.3390/polym14050956
Nepal S, Das B, Das MK, Sarkar MD, Urbańska M, Czerwiński M. Static Permittivity and Electro-Optical Properties of Bi-Component Orthoconic Antiferroelectric Liquid Crystalline Mixtures Targeted for Polymer Stabilized Sensing Systems. Polymers. 2022; 14(5):956. https://doi.org/10.3390/polym14050956
Chicago/Turabian StyleNepal, Shantiram, Banani Das, Malay Kumar Das, Madhumita Das Sarkar, Magdalena Urbańska, and Michał Czerwiński. 2022. "Static Permittivity and Electro-Optical Properties of Bi-Component Orthoconic Antiferroelectric Liquid Crystalline Mixtures Targeted for Polymer Stabilized Sensing Systems" Polymers 14, no. 5: 956. https://doi.org/10.3390/polym14050956
APA StyleNepal, S., Das, B., Das, M. K., Sarkar, M. D., Urbańska, M., & Czerwiński, M. (2022). Static Permittivity and Electro-Optical Properties of Bi-Component Orthoconic Antiferroelectric Liquid Crystalline Mixtures Targeted for Polymer Stabilized Sensing Systems. Polymers, 14(5), 956. https://doi.org/10.3390/polym14050956