Integrating Boronic Esters and Anthracene into Covalent Adaptable Networks toward Stimuli-Responsive Elastomers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
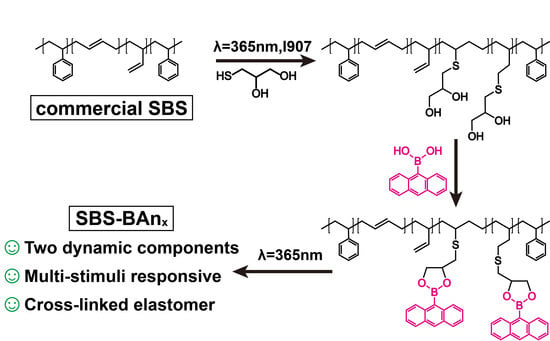
2.2. The Synthesis of Dihydroxyl Groups Functionalized SBS
2.3. Preparation of Cross-Linked SBS Networks (SBS–Banx)
2.4. Reprocessing of SBS–BAnx
2.5. The Calculation of Gel Fraction and Swelling Ratio
2.6. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Yang, H. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgorski, M.; Fairbanks, B.D.; Kirkpatrick, B.E.; McBride, M.; Martinez, A.; Dobson, A.; Bongiardina, N.J.; Bowman, C.N. Toward Stimuli-Responsive Dynamic Thermosets through Continuous Development and Improvements in Covalent Adaptable Networks (CANs). Adv. Mater. 2020, 32, 1906876. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gonzalez, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The light-controlling of temperature-responsivity in stimuli-responsive polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Zola, R.S.; Bisoyi, H.K.; Wang, H.; Urbas, A.M.; Bunning, T.J.; Li, Q. Dynamic Control of Light Direction Enabled by Stimuli-Responsive Liquid Crystal Gratings. Adv. Mater. 2019, 31, 1806172. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Gao, R.; Fang, X.; Yan, D. Recent developments in stimuli-responsive luminescent films. J. Mater. Chem. C 2019, 7, 3399–3412. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-responsive polymers for sensing and actuation. Mater. Horiz. 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Jung, K.; Li, A.; Liu, J.; Boyer, C. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Prog. Polym. Sci. 2019, 99, 101164. [Google Scholar] [CrossRef]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem. Eur. J. 2015, 21, 13164–13174. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.; Yin, J.; Jiang, X. Smart patterned surface with dynamic wrinkles. Acc. Chem. Res. 2019, 52, 1025–1035. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, L.; Liu, Y.; Xu, Z.; Yin, J.; Ge, D.; Jiang, X. Dynamic Structural Color from Wrinkled Thin Films. Adv. Opt. Mater. 2020, 8, 2000234. [Google Scholar] [CrossRef]
- Li, F.; Hou, H.; Yin, J.; Jiang, X. Near-infrared light–responsive dynamic wrinkle patterns. Sci. Adv. 2018, 4, 5762. [Google Scholar] [CrossRef] [Green Version]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef] [Green Version]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: From Old Chemistry to Modern Day Innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmed, N.; Rabnawaz, M. Covalent Adaptable Network and Self-Healing Materials: Current Trends and Future Prospects in Sustainability. Polymers 2020, 12, 2027. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, Y.; Ji, Y. Polymer actuators based on covalent adaptable networks. Polym. Chem. 2020, 11, 5297–5320. [Google Scholar] [CrossRef]
- Tsuruoka, A.; Takahashi, A.; Aoki, D.; Otsuka, H. Fusion of different crosslinked polymers based on dynamic disulfide exchange. Angew. Chem. Int. Ed. 2020, 59, 4294–4298. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Imbernon, L.; Oikonomou, E.; Norvez, S.; Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym. Chem. 2015, 6, 4271–4278. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Wu, X.; Guan, Q.; Chen, S.; Sun, L.; Guo, Y.; Wang, S.; Song, J.; Jeffries, E.M.; et al. A highly efficient self-healing elastomer with unprecedented mechanical properties. Adv. Mater. 2019, 31, 1901402. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Liu, W.; Li, P.; Liu, J.; Liu, C.; Zhang, J.; Zhao, N.; Xu, J. Recyclable polybutadiene elastomer based on dynamic imine bond. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2011–2018. [Google Scholar] [CrossRef]
- Lessard, J.J.; Garcia, L.F.; Easterling, C.P.; Sims, M.B.; Bentz, K.C.; Arencibia, S.; Savin, D.A.; Sumerlin, B.S. Catalyst-free vitrimers from vinyl polymers. Macromolecules 2019, 52, 2105–2111. [Google Scholar] [CrossRef]
- Denissen, W.; De Baere, I.; Van Paepegem, W.; Leibler, L.; Winne, J.; Du Prez, F.E. Vinylogous urea vitrimers and their application in fiber reinforced composites. Macromolecules 2018, 51, 2054–2064. [Google Scholar] [CrossRef] [Green Version]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, Q.; Liu, Y.; Chen, Y.; Guo, B.; Zhang, L. Uniaxial stretching-induced alignment of carbon nanotubes in cross-linked elastomer enabled by dynamic cross-link reshuffling. ACS Macro Lett. 2019, 8, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Cromwell, O.R.; Chung, J.; Guan, Z. Malleable and Self-Healing Covalent Polymer Networks through Tunable Dynamic Boronic Ester Bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bao, C.; Xie, P.; Guo, Z.; Sun, J. Solution-Processable and Thermostable Super-Strong Poly(aryl ether ketone) Supramolecular Thermosets Cross-Linked with Dynamic Boroxines. Adv. Funct. Mater. 2021, 31, 2103061. [Google Scholar] [CrossRef]
- Bapat, A.P.; Sumerlin, B.S.; Sutti, A. Bulk network polymers with dynamic B–O bonds: Healable and reprocessable materials. Mater. Horiz. 2020, 7, 694–714. [Google Scholar] [CrossRef]
- Brooks, W.L.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic acid building blocks: Tools for self assembly. Chem. Commun. 2011, 47, 1124–1150. [Google Scholar] [CrossRef]
- Antonio, J.P.M.; Russo, R.; Carvalho, C.P.; Cal, P.M.S.D.; Gois, P.M.P. Boronic acids as building blocks for the construction of therapeutically useful bioconjugates. Chem. Soc. Rev. 2019, 48, 3513–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röttger, M.; Domenech, T.; Van der Weegen, R.; Breuillac, A.; Nicolaÿ, R.; Leibler, L. High-performance vitrimers from commodity thermoplastics through dioxaborolane metathesis. Science 2017, 356, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Droesbeke, M.; Nicolay, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Chemical control of the viscoelastic properties of vinylogous urethane vitrimers. Nat. Commun. 2017, 8, 14857. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ma, Y.; Xiang, Y.; Shen, X.; Shi, Z.; Gao, J. Integrating Boronic Esters and Anthracene into Covalent Adaptable Networks toward Stimuli-Responsive Elastomers. Polymers 2022, 14, 1104. https://doi.org/10.3390/polym14061104
Liu Z, Ma Y, Xiang Y, Shen X, Shi Z, Gao J. Integrating Boronic Esters and Anthracene into Covalent Adaptable Networks toward Stimuli-Responsive Elastomers. Polymers. 2022; 14(6):1104. https://doi.org/10.3390/polym14061104
Chicago/Turabian StyleLiu, Zhiyong, Youwei Ma, Yixin Xiang, Xianrong Shen, Zixing Shi, and Jiangang Gao. 2022. "Integrating Boronic Esters and Anthracene into Covalent Adaptable Networks toward Stimuli-Responsive Elastomers" Polymers 14, no. 6: 1104. https://doi.org/10.3390/polym14061104
APA StyleLiu, Z., Ma, Y., Xiang, Y., Shen, X., Shi, Z., & Gao, J. (2022). Integrating Boronic Esters and Anthracene into Covalent Adaptable Networks toward Stimuli-Responsive Elastomers. Polymers, 14(6), 1104. https://doi.org/10.3390/polym14061104