Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis and Thiolation of rGO
2.2. Synthesis of Graphitic Carbon Nitride Nanosheets (g-C3N4)
2.3. Synthesis of Thiolated Graphitic Carbon Nitride Nanosheets (HS-g-C3N4)
2.4. Fabrication of rGO@g-C3N4 and HS-rGO@g-C3N4
2.5. Synthesis of AgNPs onto the Surface of HS-rGO2%@g-C3N4
2.6. Characterisations
2.7. Hydrogen Production
2.8. Photocatalytic Degradation of Ciprofloxacin Antibiotics
3. Results and Discussion
3.1. Structural Analysis
3.2. Optical Properties
3.3. Surface Chemical State Investigation
3.4. Surface Morphology
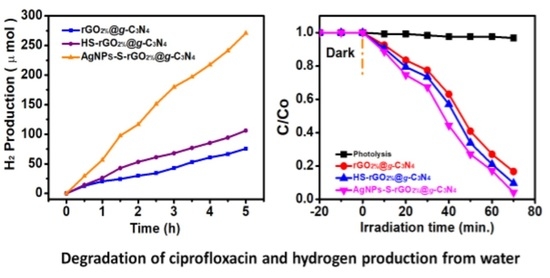
3.5. Hydrogen Production and Proposed Mechanism for AgNPs-S-rGO2%@g-C3N4
3.6. Photocatalytic CIP Degradation
Effect of AgNPs-S-rGO2%@g-C3N4 Dosage over CIP Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 14, 260–269. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Sivanantham, A.; Hwang, S.W.; Surendran, S.; Sim, U.; Cho, I.S. Facile fabrication of nanotubular heterostructure for enhanced photoelectrochemical performance. Ceram. Int. 2021, 47, 3972–3977. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Rodene, D.D.; Gupta, R.B. Recent advancements in semiconductor materials for photoelectrochemical water splitting for hydrogen production using visible light. Renew. Sustain. Energy Rev. 2018, 89, 228–248. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, Y.-S.; Huang, H.; Su, P.-J.; Perng, T.-P.; Chen, L.-J. Photocatalytic enhancement of hydrogen production in water splitting under simulated solar light by band gap engineering and localized surface plasmon resonance of ZnxCd1-xS nanowires decorated by Au nanoparticles. Nano Energy 2020, 67, 104225. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Hwang, S.W.; Kim, H.; Sim, Y.; Surendran, S.; Sim, U.; Cho, I.S. (020)-Textured tungsten trioxide nanostructure with enhanced photoelectrochemical activity. J. Catal. 2020, 389, 328–336. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, H.Y. Integration of Cobalt Metal-Organic Frameworks into an Interpenetrated Prussian Blue Analogue to Derive Dual Metal-Organic Framework-Assisted Cobalt Iron Derivatives for Enhancing Electrochemical Total Water Splitting. J. Phys. Chem. C 2020, 124, 14465–14476. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/graphitic carbon nitride-based ternary nanohybrids: Synthesis methods, properties, and applications for photocatalytic hydrogen production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Bouyarmane, H.; El Bekkali, C.; Labrag, J.; Es-saidi, I.; Bouhnik, O.; Abdelmoumen, H.; Laghzizil, A.; Nunzi, J.M.; Robert, D. Photocatalytic degradation of emerging antibiotic pollutants in waters by TiO2/Hydroxyapatite nanocomposite materials. Surf. Interfaces 2021, 24, 101155. [Google Scholar] [CrossRef]
- Ding, Q.; Lam, F.L.Y.; Hu, X. Complete degradation of ciprofloxacin over g-C3N4-iron oxide composite via heterogeneous dark Fenton reaction. J. Environ. Manag. 2019, 244, 23–32. [Google Scholar] [CrossRef]
- Das, K.K.; Patnaik, S.; Mansingh, S.; Behera, A.; Mohanty, A.; Acharya, C.; Parida, K.M. Enhanced photocatalytic activities of polypyrrole sensitized zinc ferrite/graphitic carbon nitride n-n heterojunction towards ciprofloxacin degradation, hydrogen evolution and antibacterial studies. J. Colloid Interface Sci. 2020, 561, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Fu, L.; Sun, Z.; Ou, M.; Zhao, S.; Chen, Y.; Yu, F.; Wu, Y. A defective g-C3N4/RGO/TiO2 composite from hydrogen treatment for enhanced visible-light photocatalytic H2 production. Nanoscale 2020, 12, 22030–22035. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Fan, B.; Feng, Y.; Chen, H.; Jiang, F.; Wang, X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018, 353, 147–156. [Google Scholar] [CrossRef]
- Sun, J.; Yang, S.; Liang, Z.; Liu, X.; Qiu, P.; Cui, H.; Tian, J. Two-dimensional/one-dimensional molybdenum sulfide (MoS2) nanoflake/graphitic carbon nitride (g-C3N4) hollow nanotube photocatalyst for enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2020, 567, 300–307. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials 2019, 13, 114. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Huang, W.-Q.; Wang, L.-L.; Tian, Z.-A.; Hu, W.; Ma, Y.; Wang, X.; Pan, A.; Huang, G.-F. Insights into Enhanced Visible-Light Photocatalytic Hydrogen Evolution of g-C3N4 and Highly Reduced Graphene Oxide Composite: The Role of Oxygen. Chem. Mater. 2015, 27, 1612–1621. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Park, S.-J.; Park, M.; Kim, H.-Y. Synthesis and characterization of reduced graphene oxide decorated with CeO2-doped MnO2 nanorods for supercapacitor applications. J. Colloid Interface Sci. 2017, 494, 338–344. [Google Scholar] [CrossRef]
- Jilani, A.; Rehman, G.U.; Ansari, M.O.; Othman, M.H.D.; Hussain, S.Z.; Dustgeer, M.R.; Darwesh, R. Sulfonated polyaniline-encapsulated graphene@graphitic carbon nitride nanocomposites for significantly enhanced photocatalytic degradation of phenol: A mechanistic study. New J. Chem. 2020, 44, 19570–19580. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.; Smith, S.C.; Chen, Z.; Lu, G.Q.; Cheng, H.-M. Unique Electronic Structure Induced High Photoreactivity of Sulfur-Doped Graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Raheman Ar, S.; Wilson, H.M.; Momin, B.M.; Annapure, U.S.; Jha, N. CdSe quantum dots modified thiol functionalized g-C3N4: Intimate interfacial charge transfer between 0D/2D nanostructure for visible light H2 evolution. Renew. Energy 2020, 158, 431–443. [Google Scholar] [CrossRef]
- Jiao, J.; Wan, J.; Ma, Y.; Wang, Y. Facile formation of silver nanoparticles as plasmonic photocatalysts for hydrogen production. RSC Adv. 2016, 6, 106031–106034. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled synthesis of Ag nanoparticles with different morphologies and their antibacterial properties. Mater. Sci. Eng. C 2013, 33, 397–404. [Google Scholar] [CrossRef]
- Jaiswal, A.; Pal, S.; Kumar, A.; Prakash, R. Metal free triad from red phosphorous, reduced graphene oxide and graphitic carbon nitride (red P-rGO-g-C3N4) as robust electro-catalysts for hydrogen evolution reaction. Electrochim. Acta 2020, 338, 135851. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Hezam, A.; Qahtan, T.F.; Al-Aswad, A.H.; Gondal, M.A.; Drmosh, Q.A. Laser-assisted synthesis of Z-scheme TiO2/rGO/g-C3N4 nanocomposites for highly enhanced photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 534, 147578. [Google Scholar] [CrossRef]
- Pan, J.; Wang, B.; Dong, Z.; Zhao, C.; Jiang, Z.; Song, C.; Wang, J.; Zheng, Y.; Li, C. The 2D RGO-NiS2 dual co-catalyst synergistic modified g-C3N4 aerogel towards enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 19942–19952. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Kumar, R.; Alshahrie, A.; Ismail, A.F.; Khan, I.U.; Sajith, V.K.; Barakat, M.A. Facile spectroscopic approach to obtain the optoelectronic properties of few-layered graphene oxide thin films and their role in photocatalysis. New J. Chem. 2017, 41, 14217–14227. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Monothiolation and Reduction of Graphene Oxide via One-Pot Synthesis: Hybrid Catalyst for Oxygen Reduction. ACS Nano 2015, 9, 4193–4199. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Jilani, A.; Ansari, M.O.; Rehman, G.u.; Shakoor, M.B.; Hussain, S.Z.; Othman, M.H.D.; Ahmad, S.R.; Dustgeer, M.R.; Alshahrie, A. Phenol removal and hydrogen production from water: Silver nanoparticles decorated on polyaniline wrapped zinc oxide nanorods. J. Ind. Eng. Chem. 2022, in press. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Hussain, S.Z.; Jilani, A.; Zhang, H.; Ansari, T.M.; Hussain, I. Magnetic Hierarchically Macroporous Emulsion-Templated Poly(acrylic acid)–Iron Oxide Nanocomposite Beads for Water Remediation. Langmuir 2019, 35, 8996–9003. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liu, Q.; Zhu, G.; Wei, X.; Bai, J.; Xuan, L.; Wang, H. Sonication assisted preparation of graphene oxide/graphitic-C3N4 nanosheet hybrid with reinforced photocurrent for photocatalyst applications. Dalton Trans. 2014, 43, 6295–6299. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Liu, S.-H.; Tang, W.-T.; Chou, P.-H. Microwave-assisted synthesis of triple 2D g-C3N4/Bi2WO6/rGO composites for ibuprofen photodegradation: Kinetics, mechanism and toxicity evaluation of degradation products. Chem. Eng. J. 2020, 387, 124098. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Huang, J.; Du, Z.; Hong, X.; Chen, X.; Hu, X.; Wang, X. Enhanced photocatalytic nitrogen fixation of Ag/B-doped g-C3N4 nanosheets by one-step in-situ decomposition-thermal polymerization method. Appl. Catal. A Gen. 2020, 601, 117647. [Google Scholar] [CrossRef]
- Amendola, V. Surface plasmon resonance of silver and gold nanoparticles in the proximity of graphene studied using the discrete dipole approximation method. Phys. Chem. Chem. Phys. 2016, 18, 2230–2241. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, Y.; Jia, J.; Zhang, L. Semiconducting optical properties of silver/silica mesoporous composite. Appl. Phys. Lett. 1998, 73, 2709–2711. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, X.; Tang, Y.; Liu, C.; Luo, S. Efficient Photocatalytic Hydrogen Evolution and CO2 Reduction: Enhanced Light Absorption, Charge Separation, and Hydrophilicity by Tailoring Terminal and Linker Units in g-C3N4. ACS Appl. Mater. Interfaces 2020, 12, 19607–19615. [Google Scholar] [CrossRef]
- Hu, K.; Yao, M.; Yang, Z.; Xiao, G.; Zhu, L.; Zhang, H.; Liu, R.; Zou, B.; Liu, B. Pressure tuned photoluminescence and band gap in two-dimensional layered g-C3N4: The effect of interlayer interactions. Nanoscale 2020, 12, 12300–12307. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Rodríguez-Castellón, E.; Bandosz, T.J. Photosensitivity of g-C3N4/S-doped carbon composites: Study of surface stability upon exposure to CO2 and/or water in ambient light. J. Mater. Chem. A 2017, 5, 24880–24891. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B Environ. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Tang, Z.-R.; Xu, Y.-J. Toward Improving the Graphene–Semiconductor Composite Photoactivity via the Addition of Metal Ions as Generic Interfacial Mediator. ACS Nano 2014, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xu, T.; Chen, G.; Yang, S.; Liu, H.; Xue, Q. Preparation and characterization of electrochemically deposited carbon nitride films on silicon substrate. J. Phys. D Appl. Phys. 2004, 37, 907–913. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Zhang, Y.; Zou, W.; Luo, Y.; Dong, L.; Gao, B. Facile one-step synthesis of graphitic carbon nitride-modified biochar for the removal of reactive red 120 through adsorption and photocatalytic degradation. Biochar 2019, 1, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Yu, J. Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 72–99. [Google Scholar] [CrossRef]
- John, F.; William, F.; Peter, E.; Kenneth, D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Wang, H.; Feng, D. The anti-seizure effect of Ag nanoparticles additive in multialkylated cyclopentanes oil under vacuum condition. Tribol. Int. 2012, 55, 1–6. [Google Scholar] [CrossRef]
- Kumar, R.; Rashid, J.; Barakat, M.A. Zero valent Ag deposited TiO2 for the efficient photocatalysis of methylene blue under UV-C light irradiation. Colloids Interface Sci. Commun. 2015, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.-J.; Yang, Y.; Li, Z.; Chen, D.; Wu, S.; Fang, G.; Bai, W.; Ding, M.; Yang, L.-X.; Cao, D.-P.; et al. Promoting Charge Separation in g-C3N4/Graphene/MoS2 Photocatalysts by Two-Dimensional Nanojunction for Enhanced Photocatalytic H2 Production. ACS Appl. Energy Mater. 2018, 1, 1400–1407. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 2015, 13, 757–770. [Google Scholar] [CrossRef]
- Lewandowska-Andralojc, A.; Malolepszy, A.; Stritt, A.; Grohmann, A. Modification of eosin Y and cobalt molecular catalyst system with reduced graphene oxide for enhanced photocatalytic hydrogen production. Catal. Sci. Technol. 2020, 10, 4693–4702. [Google Scholar] [CrossRef]
- Das, S.; Dutta, A.; Bera, R.; Patra, A. Ultrafast carrier dynamics in 2D-2D hybrid structures of functionalized GO and CdSe nanoplatelets. Phys. Chem. Chem. Phys. 2019, 21, 15568–15575. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ju, P.; Li, J.; Zhao, Y.; Han, X.; Hao, Z. Facile Synthesis of MoS2/g-C3N4/GO Ternary Heterojunction with Enhanced Photocatalytic Activity for Water Splitting. ACS Sustain. Chem. Eng. 2017, 5, 7878–7886. [Google Scholar] [CrossRef]
- Xu, X.; Si, Z.; Liu, L.; Wang, Z.; Chen, Z.; Ran, R.; He, Y.; Weng, D. CoMoS2/rGO/C3N4 ternary heterojunctions catalysts with high photocatalytic activity and stability for hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2018, 435, 1296–1306. [Google Scholar] [CrossRef]
- Jo, W.-K.; Selvam, N.C.S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2 production and pollutant degradation. Chem. Eng. J. 2017, 317, 913–924. [Google Scholar] [CrossRef]
- Rogachev, A.A.; Yarmolenko, M.A.; Rogachou, A.V.; Tapalski, D.V.; Liu, X.; Gorbachev, D.L. Morphology and structure of antibacterial nanocomposite organic–polymer and metal–polymer coatings deposited from active gas phase. RSC Adv. 2013, 3, 11226–11233. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Akbari, S.; Moussavi, G.; Giannakis, S. Efficient photocatalytic degradation of ciprofloxacin under UVA-LED, using S,N-doped MgO nanoparticles: Synthesis, parametrization and mechanistic interpretation. J. Mol. Liq. 2021, 324, 114831. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing Parameters in the Photocatalytic Degradation of Organic Effluent via Nanometal Oxide Catalyst: A Review. Indian J. Mater. Sci. 2015, 2015, 601827. [Google Scholar] [CrossRef]














| Binary Photocatalysts | Additive Co-Catalyst | Condition of Sacrificial Agent | Radiation Source | Maximum Hydrogen Yield (µmol h−1 g−1) | Reference with Year |
|---|---|---|---|---|---|
| Thiolated g-C3N4/rGO | Ag | Triethanolamine 15% | 300 W Xenon lamp | 3772.5 | This work |
| g-C3N4/rGO | MoS2 | Triethanolamine 0.1% | 300 W Xenon lamp | 317 | [50] 2018 |
| g-C3N4/rGO | MoS2 | Sodium sulfite 0.25 Molar | 450 Xenon lamp | 1650 | [54] 2017 |
| g-C3N4/rGO | NiS2 | Triethanolamine 1% | 300 W Xenon lamp | 1555.34 | [25] 2019 |
| g-C3N4/rGO | CoMoS2 | Triethanolamine 1% | 300 W Xenon lamp | 684 | [55] 2018 |
| g-C3N4/rGO | CdS | Lactic acid 10% | 350 W Xenon lamp | 1000.5 | [56] 2017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jilani, A.; Hussain, S.Z.; Melaibari, A.A.; Abu-Hamdeh, N.H. Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation. Polymers 2022, 14, 1290. https://doi.org/10.3390/polym14071290
Jilani A, Hussain SZ, Melaibari AA, Abu-Hamdeh NH. Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation. Polymers. 2022; 14(7):1290. https://doi.org/10.3390/polym14071290
Chicago/Turabian StyleJilani, Asim, Syed Zajif Hussain, Ammar A. Melaibari, and Nidal H. Abu-Hamdeh. 2022. "Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation" Polymers 14, no. 7: 1290. https://doi.org/10.3390/polym14071290
APA StyleJilani, A., Hussain, S. Z., Melaibari, A. A., & Abu-Hamdeh, N. H. (2022). Development and Mechanistic Studies of Ternary Nanocomposites for Hydrogen Production from Water Splitting to Yield Sustainable/Green Energy and Environmental Remediation. Polymers, 14(7), 1290. https://doi.org/10.3390/polym14071290