Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of PAN/PMMA Nanofibers
2.2. Synthesis of Mesoporous Carbon Nanofiber Web (mpCNF)
2.3. Synthesis of Cyclized-Polyacrylonitrile Modified CNF Fibers (CPAN/mpCPAN)
2.4. Synthesis of Electrodes
2.5. Material Characterization
2.6. Electrochemical Characterization
3. Results and Discussion
3.1. Surface and Structural Properties of Multi-Porous Carbon Nanofibers
3.2. Characterization of CPAN and mpCPAN Nanofibers
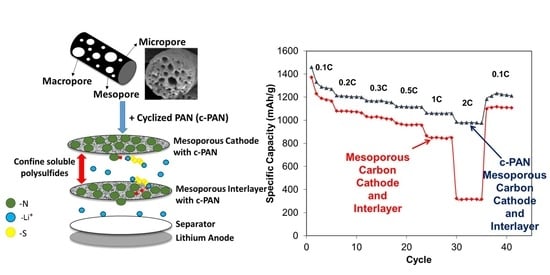
3.3. Applications of mpCNF, CPAN, and mpCPAN Nanofibers in Li–S Batteries
- mpCNF cathode and mpCNF interlayer (mpCNF-mpCNF)
- mpCNF cathode and mpCPAN interlayer (mpCNF-mpCPAN)
- mpCPAN cathode and mpCNF interlayer (mpCPAN-mpCNF)
- mpCPAN cathode and mpCPAN interlayer (mpCPAN-mpCPAN)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef] [PubMed]
- Cañas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigations of lithium-sulfur batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium-sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium-Schwefel-Batterien: Elektrochemie, Materialien und Perspektiven. Angew. Chemie 2013, 125, 13426–13441. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.P.; Joo, Y.L. Tunable Large Mesopores in Carbon Nanofiber Interlayers for High-Rate Lithium Sulfur Batteries. J. Electrochem. Soc. 2016, 163, A2745–A2756. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li–S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef]
- Schuster, J.; He, G.; Mandlmeier, B.; Yim, T.; Lee, K.T.; Bein, T.; Nazar, L.F. Spherical Ordered Mesoporous Carbon Nanoparticles with High Porosity for Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2012, 51, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium–Sulfur Battery Cathode Material with High Capacity and Cycling Stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.-Z.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q.; Li, B. The formation of strong-couple interactions between nitrogen-doped graphene and sulfur/lithium (poly)sulfides in lithium-sulfur batteries. 2D Mater. 2015, 2, 014011. [Google Scholar] [CrossRef]
- Li, F.; Su, Y.; Zhao, J. Shuttle inhibition by chemical adsorption of lithium polysulfides in B and N co-doped graphene for Li–S batteries. Phys. Chem. Chem. Phys. 2016, 18, 25241–25248. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S Batteries Using Amine-Functionalized Carbon Nanotubes in the Cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef]
- Pang, Q.; Tang, J.; Huang, H.; Liang, X.; Hart, C.; Tam, K.C.; Nazar, L.F. A Nitrogen and Sulfur Dual-Doped Carbon Derived from Polyrhodanine@Cellulose for Advanced Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 6021–6028. [Google Scholar] [CrossRef]
- Seh, Z.W.; Wang, H.; Hsu, P.-C.; Zhang, Q.; Li, W.; Zheng, G.; Yao, H.; Cui, Y. Facile synthesis of Li2S–polypyrrole composite structures for high-performance Li2S cathodes. Energy Environ. Sci. 2014, 7, 672. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Nie, H.; Gu, C.; Hua, W.; Xu, X.; Chen, X.; Chen, Y.; Huang, S. A lightweight multifunctional interlayer of sulfur–nitrogen dual-doped graphene for ultrafast, long-life lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 15343–15352. [Google Scholar] [CrossRef]
- Zeng, Q.; Leng, X.; Wu, K.-H.; Gentle, I.R.; Wang, D.-W. Electroactive cellulose-supported graphene oxide interlayers for Li–S batteries. Carbon N. Y. 2015, 93, 611–619. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon Nanofibers Prepared via Electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, M.; Qin, X.; Wu, J.; Han, W.; Liang, G.; Zhou, D.; He, Y.-B.; Li, B.; Kang, F. Cyclized-polyacrylonitrile modified carbon nanofiber interlayers enabling strong trapping of polysulfides in lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 12973–12980. [Google Scholar] [CrossRef]
- Lee, J.; Ko, B.; Kang, J.; Chung, Y.; Kim, Y.; Halim, W.; Lee, J.H.; Joo, Y.L. Facile and scalable fabrication of highly loaded sulfur cathodes and lithium–sulfur pouch cells via air-controlled electrospray. Mater. Today Energy 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Singhal, R.; Chung, S.H.; Manthiram, A.; Kalra, V. A free-standing carbon nanofiber interlayer for high-performance lithium-sulfur batteries. J. Mater. Chem. A 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Chen, C.-F.; Mistry, A.; Mukherjee, P.P. Probing Impedance and Microstructure Evolution in Lithium–Sulfur Battery Electrodes. J. Phys. Chem. C 2017, 121, 21206–21216. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L.; Hendrickson, K.E.; Tu, Z.; Archer, L.A. Metal–Sulfur Battery Cathodes Based on PAN–Sulfur Composites. J. Am. Chem. Soc. 2015, 137, 12143–12152. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Bin Wu, H.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. Enhancing lithium–sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-F.; Zhang, X.-D.; Liang, H.-W.; Kong, M.; Guan, Q.-F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Appendix, J. Line Positions in Numerical Order. In Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992; pp. 257–259. [Google Scholar]
- Hou, T.-Z.; Chen, X.; Peng, H.-J.; Huang, J.-Q.; Li, B.-Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef] [PubMed]














| Multi-Porous Carbon Nanofibers | BET Surface Area (m2/g) | Mesopore Volume/Total Volume (cm3/g) | |
|---|---|---|---|
| PAN/PMMA (59:41) | 0 N | 1284 | 0.80 |
| 0.62 N | 376 | 0.77 | |
| 1.12 N | 224 | 0.80 | |
| 1.28 N | 420 | 0.81 | |
| Ceramic pressure load 1.28N | PAN/PMMA (56:44) | 456 | 0.79 |
| PAN/PMMA (77:23) | 547 | 0.84 | |
| PAN/PMMA (80:20) | 126 | 0.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Won, J.S.; An, M.; Zhang, R.; Lee, J.H.; Lee, S.G.; Joo, Y.L. Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium–Sulfur Batteries. Polymers 2022, 14, 1342. https://doi.org/10.3390/polym14071342
Sarkar S, Won JS, An M, Zhang R, Lee JH, Lee SG, Joo YL. Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium–Sulfur Batteries. Polymers. 2022; 14(7):1342. https://doi.org/10.3390/polym14071342
Chicago/Turabian StyleSarkar, Snatika, Jong Sung Won, Meichun An, Rui Zhang, Jin Hong Lee, Seung Goo Lee, and Yong Lak Joo. 2022. "Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium–Sulfur Batteries" Polymers 14, no. 7: 1342. https://doi.org/10.3390/polym14071342
APA StyleSarkar, S., Won, J. S., An, M., Zhang, R., Lee, J. H., Lee, S. G., & Joo, Y. L. (2022). Tailoring Mesopores and Nitrogen Groups of Carbon Nanofibers for Polysulfide Entrapment in Lithium–Sulfur Batteries. Polymers, 14(7), 1342. https://doi.org/10.3390/polym14071342