Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reclystallization of PLA, PLA-co-PEAd and PLA-co-PBAd
2.3. Preparation of Melatonin Modified-Release Tablets
2.4. Tablet Uniformity Tests
2.5. In Vitro Dissolution Studies
2.6. Methods to Compare Dissolution Profiles
2.7. Attenuated Total Reflectance Infrared Spectroscopy (ATR-FTIR)
2.8. X-ray Powder Diffraction (XRD)
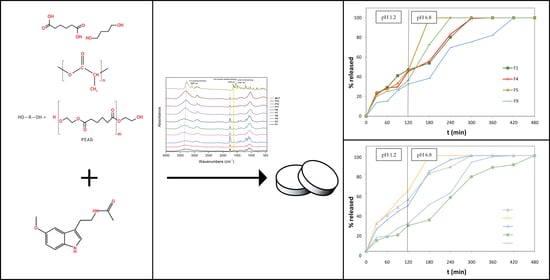
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, J. Melatonin and human rhythms. Chronobiol. Int. 2006, 23, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.W.; Sugden, D. Effects of melatonin on sleep and neurochemistry in the rat. Br. J. Pharmacol. 1982, 76, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, K.; Van Den Heuvel, C.; Dawson, D. Day-time melatonin administration: Effects on core temperature and sleep onset latency. J. Sleep Res. 1996, 5, 150–154. [Google Scholar] [CrossRef]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Dedeloudi, A.; Konstantinidou, S.K.; Papanastasiou, I.P. Pineal hormone melatonin as an adjuvant treatment for COVID-19 (Review). Int. J. Mol. Med. 2021, 47, 47. [Google Scholar] [CrossRef]
- Cross, K.M.; Landis, D.M.; Sehgal, L.; Payne, J.D. Melatonin for the Early Treatment of COVID-19: A Narrative Review of Current Evidence and Possible Efficacy. Endocr. Pract. 2021, 27, 850–855. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Mukherjee, D.; Roy, S.G.; Bandyopadhyay, A.; Chattopadhyay, A.; Basu, A.; Mitra, E.; Ghosh, A.K.; Reiter, R.J.; Bandyopadhyay, D. Melatonin protects against isoproterenol-induced myocardial injury in the rat: Antioxidative mechanisms. J. Pineal Res. 2010, 48, 251–262. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Barcelo, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Clinical uses of melatonin: Evaluation of human trials. Curr. Med. Chem. 2010, 17, 2070–2095. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R.; Godson, C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharmacol. Sci. 1996, 17, 100–102. [Google Scholar] [CrossRef]
- Reppert, S.M. Melatonin Receptors: Molecular Biology of a New Family of G Protein-Coupled Receptors. J. Biol. Rhythms 1997, 12, 528–531. [Google Scholar] [CrossRef]
- Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Han, G.W.; Patel, N.; Huang, X.P.; Batyuk, A.; Gati, C.; Slocum, S.T.; Li, C.; et al. XFEL structures of the human MT2 melatonin receptor reveal the basis of subtype selectivity. Nature 2019, 569, 289–292. [Google Scholar] [CrossRef]
- Izuhara, M.; Kawano, K.; Otsuki, K.; Hashioka, S.; Inagaki, M. Prompt improvement of difficulty with sleep initiation and waking up in the morning and daytime somnolence by combination therapy of suvorexant and ramelteon in delayed sleep-wake phase disorder: A case series of three patients. Sleep Med. 2021, 80, 100–104. [Google Scholar] [CrossRef]
- Sanches, M.; Quevedo, J.; Soares, J.C. New agents and perspectives in the pharmacological treatment of major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110157. [Google Scholar] [CrossRef]
- Vlachou, M.; Ioannidou, V.; Vertzoni, M.; Tsotinis, A.; Afroudakis, P.; Sugden, D. Controlled release from solid pharmaceutical formulations of two nalkanoyl-4-methoxybicyclo[4.2.0]octa-1,3,5-trien-7-ethanamines with melatoninergic activity. Lett. Drug Des. Discov. 2015, 12, 259–262. [Google Scholar] [CrossRef]
- Vlachou, M.; Papamichael, M.; Siamidi, A.; Fragouli, I.; Afroudakis, P.A.; Kompogennitaki, R.; Dotsikas, Y. Comparative in vitro controlled release studies on the chronobiotic hormone melatonin from cyclodextrins-containing matrices and cyclodextrin: Melatonin complexes. Int. J. Mol. Sci. 2017, 18, 1641. [Google Scholar] [CrossRef] [Green Version]
- Vlachou, M.; Tragou, T.; Siamidi, A.; Kikionis, S.; Chatzianagnostou, A.L.; Mitsopoulos, A.; Ioannou, E.; Roussis, V.; Tsotinis, A. Modified in vitro release of the chronobiotic hormone melatonin from matrix tablets based on the marine sulfated polysaccharide ulvan. J. Drug Deliv. Sci. Technol. 2018, 44, 41–48. [Google Scholar] [CrossRef]
- Martarelli, D.; Casettari, L.; Shalaby, K.S.; Soliman, M.E.; Cespi, M.; Bonacucina, G.; Fagioli, L.; Perinelli, D.R.; Lam, J.K.; Palmieri, G.F. Optimization of Melatonin Dissolution from Extended Release Matrices Using Artificial Neural Networking. Curr. Drug Deliv. 2016, 13, 565–573. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, S.P.; Khanna, R. Modified release bi-layered tablet of melatonin using -cyclodextrin. Pharmazie 2008, 58, 642–644. [Google Scholar]
- Luthringer, R.; Muzet, M.; Zisapel, N.; Staner, L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int. Clin. Psychopharmacol. 2009, 24, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Ye, M.; Kim, S.; Park, K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release 2010, 146, 241–260. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Karava, V.; Siamidi, A.; Vlachou, M.; Christodoulou, E.; Zamboulis, A.; Bikiaris, D.N.; Kyritsis, A.; Klonos, P.A. Block copolymers based on poly(butylene adipate) and poly(l-lactic acid) for biomedical applications: Synthesis, structure and thermodynamical studies. Soft Matter 2021, 17, 2439–2453. [Google Scholar] [CrossRef]
- Zorba, T.; Chrissafis, K.; Paraskevopoulos, K.M.; Bikiaris, D.N. Synthesis, characterization and thermal degradation mechanism of three poly (alkylene adipate)s: Comparative study. Polym. Degrad. Stab. 2007, 92, 222–230. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Barmpalexis, P.; Lazaridou, M.; Papageorgiou, G.Z.; Koutris, E.; Karavas, E.; Kostoglou, M.; Bikiaris, D.N. Controlled release formulations of risperidone antipsychotic drug in novel aliphatic polyester carriers: Data analysis and modelling. Eur. J. Pharm. Biopharm. 2015, 94, 473–484. [Google Scholar] [CrossRef]
- Brunner, C.T.; Baran, E.T.; Pinho, E.D.; Reis, R.L.; Neves, N.M. Performance of biodegradable microcapsules of poly (butylene succinate), poly (butylene succinate-co-adipate) and poly (butylene terephthalate-co-adipate) as drug encapsulation systems. Colloids Surf. B Biointerfaces 2011, 84, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revision of Monograph on Tablets: Final Text for Addition to the International Pharmacopoeia. Available online: https://www.who.int/medicines/publications/pharmacopoeia/Tabs-GeneralMono-rev-FINAL_31032011.pdf (accessed on 8 March 2022).
- Khan, K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT). Int. J. Pharm. 1993, 97, 93–100. [Google Scholar] [CrossRef]
- Rinaki, E.; Dokoumetzidis, A.; Macheras, P. The mean dissolution time depends on the dose/solubility ratio. Pharm. Res. 2003, 20, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Korsmeyer, W.R.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Banas, A.; Banas, K.; Kalaiselvi, S.M.P.; Pawlicki, B.; Kwiatek, W.M.; Breese, M.B.H. Is it possible to find presence of lactose in pharmaceuticals?—Preliminary studies by ATR-FTIR spectroscopy and chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 280–286. [Google Scholar] [CrossRef]
- Jafari, H.; Hassanpour, M.; Akbari, A.; Rezie, J.; Gohari, G.; Reza Mahdavinia, G.; Jabbari, E. Characterization of pH-sensitive chitosan/hydroxypropyl methylcellulose composite nanoparticles for delivery of melatonin in cancer therapy. Mater. Lett. 2020, 282, 128818. [Google Scholar] [CrossRef]
- Chakraborty, S.; Paul, K.; Mallick, P.; Pradhan, S.; Das, K.; Chakrabarti, S.; Nandi, D.K.; Bhattacharjee, P. Consortia of bioactives in supercritical carbon dioxide extracts of mustard and small cardamom seeds lower serum cholesterol levels in rats: New leads for hypocholesterolaemic supplements from spices. J. Nutr. Sci. 2019, 8, e32. [Google Scholar] [CrossRef] [Green Version]
- Topal, B.; Altındal, D.Ç.; Gümüşderelioğlu, M. Melatonin/HPβCD complex: Microwave synthesis, integration with chitosan scaffolds and inhibitory effects on MG-63CELLS. Int. J. Pharm. 2015, 496, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y. Preparation of Melatonin-Loaded Zein Nanoparticles Using Supercritical CO2 Antisolvent and In Vitro Release Evaluation. Int. J. Food Eng. 2017, 13, 20170239. [Google Scholar] [CrossRef]
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Manjunath Kamath, S.; Subha Krishna, R.; Jaison, D.; Sridhar, K.; Kasthuri, N.; Gopinath, V.; Sivaperumal, P.; Shantanu Patil, S. Melatonin delivery from PCL scaffold enhances glycosaminoglycans deposition in human chondrocytes—Bioactive scaffold model for cartilage regeneration. Process Biochem. 2020, 99, 36–47. [Google Scholar] [CrossRef]
- Ghosh, S.; Rai, S.K.; Haldar, C.; Pandey, R.S. Synthesis, Characterization, and Evaluation of Toxicity of Melatonin-Loaded Poly (D, L-Lactic Acid) Nanoparticles (Mel-PLA-Nanoparticles) and Its Putative Use in Osteoporosis. In Innovations in Food Technology; Springer: Singapore, 2020; pp. 385–394. [Google Scholar]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Risperidone controlled release microspheres based on poly (lactic acid)-poly (propylene adipate) novel polymer blends appropriate for long acting injectable formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.K.; Haldar, C.; Vishwas, D.K.; Maiti, P. Synthesis and in vitro evaluation of melatonin entrapped PLA nanoparticles: An oxidative stress and T-cell response using golden hamster. J. Biomed. Mater. Res. A 2015, 103, 3034–3044, Erratum in J. Biomed. Mater. Res. A 2018, 106, 858. [Google Scholar] [CrossRef]
- Ghosh, S. Melatonin Loaded Biodegradable Nano-Particles and Osteoporosis: A Mini Review. Sch. Acad. J. Pharm. 2021, 6, 102–106. [Google Scholar]
- Ghosh, S. Melatonin loaded poly (D, L-lactic acid) Nano-Particles (Mel-PLA-Nano-Particles) and its alleged use in osteoporosis: Mini review. IOSR J. Pharm. Biol. Sci. 2021, 16, 51–55. [Google Scholar]
- Ghimire, M.; Hodges, L.A.; Band, J.; O’Mahony, B.; McInnes, F.J.; Mullen, A.B.; Stevens, H.N.E. In-vitro and in-vivo erosion profiles of hydroxypropylmethylcellulose (HPMC) matrix tablets. J. Control. Release 2010, 147, 70–75. [Google Scholar] [CrossRef]
- Hamed, R.; Al Baraghthi, T.; Sunoqrot, S. Correlation between the viscoelastic properties of the gel layer of swollen HPMC matrix tablets and their in vitro drug release. Pharm. Dev. Technol. 2018, 23, 838–848. [Google Scholar] [CrossRef]
- Tukaram, B.N.; Rajagopalan, I.V.; Shartchandra, P.S.I. The Effects of Lactose, Microcrystalline Cellulose and Dicalcium Phosphate on Swelling and Erosion of Compressed HPMC Matrix Tablets: Texture Analyzer. Iran. J. Pharm. Res. 2010, 9, 349–358. [Google Scholar]
- Vlachou, M.; Stavrou, G.; Siamidi, A.; Flitouri, S.; Ioannidou, V.; Mavrokordopoulos, S. N-Acetylserotonin vs. Melatonin: In vitro controlled release from hydrophilic matrix tablets. Lett. Drug Des. Discov. 2019, 16, 347–352. [Google Scholar] [CrossRef]
- Yasmin, R.; Shoaib, M.H.; Ahmed, F.R.; Qazi, F.; Ali, H.; Zafar, F. Aceclofenac fast dispersible tablet formulations: Effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics. PLoS ONE 2002, 15, e0223201. [Google Scholar] [CrossRef] [PubMed]







| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Neat PLA | 68 | 34 | 34 | ||||||||||||
| PLA/PEAd [90/10] | 68 | 34 | 34 | ||||||||||||
| PLA/PEAd [75/25] | 68 | 34 | 34 | ||||||||||||
| PLA/PBAd [90/10] | 68 | 34 | 34 | ||||||||||||
| PLA/PBAd [75/25] | 68 | 34 | 34 | ||||||||||||
| HPMC K15 | 16 | 16 | 16 | 16 | 16 | 120 | 120 | 120 | 120 | 119.5 | 16 | 16 | 16 | 16 | 16 |
| Sod.Alginate | 78 | 78 | 78 | 78 | 78 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | 8 |
| Lactose | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | 8 | 20 | 20 | 20 | 20 | 20 |
| Avicel PH 102 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 120 | 120 | 120 | 120 | 120 |
| Mg.Stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Formulations | MDT | t20% | t50% | t90% | n | Mean % D.E. |
|---|---|---|---|---|---|---|
| F1 | 170.24 | 24 | 140 | 270 | 0.49 | 70.06 |
| F2 | 127.55 | 22 | 102 | 177 | 0.58 | 78.90 |
| F3 | 136.36 | 22 | 120 | 179 | 0.63 | 77.10 |
| F4 | 142.20 | 24 | 142 | 262 | 0.52 | 69.67 |
| F5 | 133.48 | 22 | 152 | 169 | 0.52 | 77.68 |
| F6 | 192.80 | 81 | 218 | 299 | 1.21 | 59.44 |
| F7 | 151.35 | 41 | 122 | 341 | 0.89 | 67.40 |
| F8 | 171.82 | 24 | 162 | 240 | 0.38 | 69.77 |
| F9 | 193.70 | 72 | 201 | 384 | 0.65 | 58.11 |
| F10 | 158.14 | 30 | 141 | 219 | 0.42 | 72.67 |
| F11 | 140.08 | 20 | 102 | 245 | 0.46 | 76.16 |
| F12 | 115.05 | 20 | 82 | 162 | 0.60 | 81.37 |
| F13 | 141.63 | 24 | 122 | 210 | 0.53 | 76.02 |
| F14 | 208.44 | 100 | 222 | 400 | 1.09 | 54.25 |
| F15 | 179.30 | 78 | 180 | 296 | 0.98 | 62.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlachou, M.; Siamidi, A.; Anagnostopoulou, D.; Christodoulou, E.; Bikiaris, N.D. Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers. Polymers 2022, 14, 1504. https://doi.org/10.3390/polym14081504
Vlachou M, Siamidi A, Anagnostopoulou D, Christodoulou E, Bikiaris ND. Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers. Polymers. 2022; 14(8):1504. https://doi.org/10.3390/polym14081504
Chicago/Turabian StyleVlachou, Marilena, Angeliki Siamidi, Dionysia Anagnostopoulou, Evi Christodoulou, and Nikolaos D. Bikiaris. 2022. "Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers" Polymers 14, no. 8: 1504. https://doi.org/10.3390/polym14081504
APA StyleVlachou, M., Siamidi, A., Anagnostopoulou, D., Christodoulou, E., & Bikiaris, N. D. (2022). Modified Release of the Pineal Hormone Melatonin from Matrix Tablets Containing Poly(L-lactic Acid) and Its PLA-co-PEAd and PLA-co-PBAd Copolymers. Polymers, 14(8), 1504. https://doi.org/10.3390/polym14081504