Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Ingredients
2.2. Preparation of Maillard Reaction Model System
2.3. Analyses of Maillard Reaction Products (MRPs), Acrylamide, Hydroxymethylfurfural (HMF), Kinematic Viscosity, Reducing Sugar, Asparagine, and pH of Solutions
2.4. Chromaticity Testing
2.5. Statistical Analysis
3. Results
3.1. MRPs of Solutions
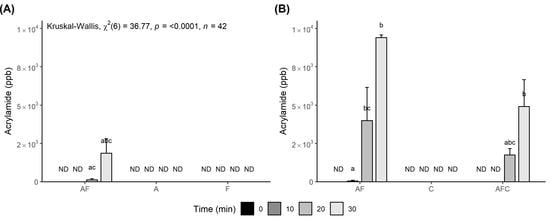
3.2. Effect of Chitosan Addition on the Formation of Acrylamide and HMF
3.3. Kinematic Viscosity, Reducing Sugar, Asparagine, and pH of Solutions
3.4. The Colorimetric Analysis of MRPs
4. Discussion
4.1. MRPs of Solutions
4.2. Effect of Chitosan Addition on the Formation of Acrylamide and HMF
4.3. Kinematic Viscosity, Reducing Sugar, Asparagine, and pH of Solutions
4.4. Chromaticity Testing of MRPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulcan, U.; Uslu, C.C.; Mutlu, C.; Arslan-Tontul, S.; Erbas, M. Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Peters, R.J.; Van Boekel, M.A. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Tornqvist, M. Acrylamide: A cooking carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Lau, B.P.Y.; Lewis, D.; Seaman, S.W. Acrylamide in foods: Occurrence, sources, and modeling. J. Agric. Food Chem. 2003, 51, 802–808. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Parker, J.K.; Balagiannis, D.P.; Higlely, J.; Smith, G.; Wedzicha, B.L.; Mottram, D.S. Kinetic model for the formation of acrylamide during the finish-frying of commercial French fries. J. Agric. Food Chem. 2012, 60, 9321–9331. [Google Scholar] [CrossRef]
- Zyzak, D.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Ebehart, L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Miallard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janckkova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active edible films fortified with natural extracts: Case study with fresh-cut apple pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Chatosan to Platform Chemicals. Master’s Thesis, Department of Energy and Environment, Tampere University, New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Sung, W.C.; Chang, Y.W.; Chou, Y.H.; Hsiao, H.I. The functional properties of chitosan-glucose-asparagine Maillard reaction products and mitigation of acrylamide formation by chitosans. Food Chem. 2018, 243, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Sung, W.C.; Chen, J.Y. Effect of different molecular weight chitosans on the mitigation of acrylamide formation and the functional properties of the resultant Maillard reaction products. Food Chem. 2016, 199, 581–589. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Shimojoh, M. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Codex. Code of Practice for the Reduction of Acrylamide in Foods (CAC/PCP 67-2009). 2009. Available online: http://www.codexalimentarius.net/download/standards/11258/CXP_067e.pdf (accessed on 5 February 2022).
- Lin, H.T.V.; Chan, D.S.; Kao, L.Y.; Sung, W.C. Effect of hydroxymethylfurfural and low-molecular-weight chitosan on formation of acrylamide and hydroxymethylfurfural during Maillard reaction in glucose and asparagine model systems. Polymers 2021, 13, 1901. [Google Scholar] [CrossRef]
- Chang, Y.W.; Zeng, X.Y.; Sung, W.C. Effect of chitooligosaccharide and different low molecular weight chitosans on the formation of acrylamide and 5-hydroxymethylfurfural and Maillard reaction products in glucose/fructose-asparagine model systems. LWT-Food Sci. Technol. 2020, 119, 108879. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Fan, T. Viscosity Measurement Using CANNON-FENSKE Viscometers. 2001. Available online: http://www.prrc.nmt.edu/groups/petrophysics/media/pdf/viscometer.pdf (accessed on 3 February 2022).
- Başkan, K.S.; Tütem, E.; Akyüz, E.; Özen, S.; Apak, R. Spectrophotometric total reducing sugars assay based on cupric reduction. Talanta 2016, 147, 162–168. [Google Scholar] [CrossRef]
- Bartolomeo, M.P.; Maisano, F. Validation of a reversed-phase HPLC method for quantitative amino acid analysis. J. Biomol. Tech. 2006, 17, 131–137. [Google Scholar]
- Kim, J.S.; Lee, Y.S. Study of Maillard reaction products derived from aqueous model systems with different peptide chain lengths. Food Chem. 2009, 116, 846–853. [Google Scholar] [CrossRef]
- Zhu, K.X.; Li, J.; Li, M.; Guo, X.N.; Peng, W.; Zhou, H.M. Functional properties of chitosan–xylose Maillard reaction products and their application to semi-dried noodle. Carbohydr. Polym. 2013, 92, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Senyuva, H.Z. Acrylamide formation is prevented by diavalent cations during the Maillard reaction. Food Chem. 2007, 103, 196–203. [Google Scholar] [CrossRef]
- Mengíbar, M.; Miralles, B.; Heras, Á. Use of soluble chitosans in Maillard reaction products with β-lactoglobulin. Emulsifying and antioxidant properties. LWT-Food Sci. Technol. 2017, 75, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Li, S.L.; Lin, J.; Chen, X.M. Effect of chitosan molecular weight on the functional properties of chitosan-maltose Maillard reaction products and their application to fresh-cut Typha latifolia L. Carbohydr. Polym. 2014, 102, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, É.; Dufossé, L.; Guérard, F. Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Gökmen, V.; Kocadağlı, T.; Göncüoğlu, N.; Mogol, B.A. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chem. 2012, 132, 168–174. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Zhang, H.; Wu, G.; Wang, L.; Qian, H.; Qi, X. Epicatechin Adducting with 5-Hydroxymethylfurfural as an Inhibitory Mechanism against Acrylamide Formation in Maillard Reactions. J. Agric. Food Chem. 2018, 66, 12536–12543. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Zweigenbaum, J.A.; Zhang, G.; Mitchell, A.E. The Influence of pH and Sodium Hydroxide Exposure Time on Glucosamine and Acrylamide Levels in California-Style Black Ripe Olives. J. Food Sci. 2017, 82, 1574–1581. [Google Scholar] [CrossRef]
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas chromatographic investigation of acrylamide formation in browning model systems. J. Agric. Food Chem. 2003, 51, 3999–4003. [Google Scholar] [CrossRef]
- Gentry, T.S.; Roberts, J.S. Formation kinetics and application of 5-hydroxymethylfurfural as a time–temperature indicator of lethality for continuous pasteurization of apple cider. Innov. Food Sci. Emerg. Technol. 2004, 5, 327–333. [Google Scholar] [CrossRef]
- Kavousi, P.; Mirhosseini, H.; Ghazali, H.; Ariffin, A.A. Formation and reduction of 5-hydroxymethylfurfural at frying temperature in model system as a function of amino acid and sugar composition. Food Chem. 2015, 182, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Locas, C.P.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2-furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Desbrieres, J. Viscosity of semiflexible chitosan solutions: Influence of concentration, temperature, and role of intermolecular interactions. Biomacromolecules 2002, 3, 342–349. [Google Scholar] [CrossRef]
- No, H.K.; Nah, J.W.; Meyers, S.P. Effect of time/temperature treatment parameters on depolymerization of chitosan. J. Appl. Polym. Sci. 2003, 87, 1890–1894. [Google Scholar] [CrossRef]
- Yan, X.; Evenocheck, H.M. Chitosan analysis using acid hydrolysis and HPLC/UV. Carbohydr. Polym. 2012, 87, 1774–1778. [Google Scholar] [CrossRef]
- Kwak, E.J.; Lim, S.I. The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef]
- Doolittle, R.F.; Falter, H.; Horn, M.J.; Kannan, K.K.; Mross, G.A.; Laursen, R.A.; Needleman, S.B.; Nieboer, E.; Reichlin, M. Advanced Methods in Protein Sequence Determination; Springer Science & Business Media: New York, NY, USA, 2012; Volume 25, Available online: https://www.springer.com/gp/book/9783662128343 (accessed on 3 February 2022).
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Dasu, V.V.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid DNS assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef]
- De Vleeschouwer, K.; Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. The kinetics of acrylamide formation/elimination in asparagine–glucose systems at different initial reactant concentrations and ratios. Food Chem. 2008, 111, 719–729. [Google Scholar] [CrossRef]
- Martins, S.I.; Van Boekel, M.A. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]








| L* | a* | b* | ΔE | |
|---|---|---|---|---|
| AFW 0 min | 100.13 ± 0.00 a | 0.42 ± 0.01 a | −0.20 ± 0.04 c | 0.48 ± 0.02 c |
| AFW 10 min | 100.13 ± 0.01 a | 0.08 ± 0.12 cd | 0.41 ± 0.20 c | 0.47 ± 0.15 c |
| AFW 20 min | 100.08 ± 0.01 bc | −2.07 ± 0.15 e | 4.26 ± 0.25 b | 4.73 ± 0.28 b |
| AFW 30 min | 98.97 ± 0.08 e | −17.10 ± 0.25 f | 47.01 ± 1.66 a | 50.04 ± 1.65 a |
| AW 0 min | 100.10 ± 0.01 ab | 0.31 ± 0.01 ab | −0.11 ± 0.01 c | 0.35 ± 0.00 c |
| AW 10 min | 100.11 ± 0.01 ab | 0.38 ± 0.03 ab | −0.19 ± 0.04 c | 0.44 ± 0.05 c |
| AW 20 min | 100.11 ± 0.01 ab | 0.41 ± 0.03 a | −0.19 ± 0.05 c | 0.46 ± 0.05 c |
| AW 30 min | 100.12 ± 0.00 ab | 0.34 ± 0.02 ab | −0.08 ± 0.06 c | 0.37 ± 0.03 c |
| FW 0 min | 100.00 ± 0.01 d | 0.12 ± 0.03 cd | −0.07 ± 0.06 c | 0.14 ± 0.05 c |
| FW 10 min | 100.05 ± 0.02 c | 0.32 ± 0.11 ab | −0.24 ± 0.15 c | 0.40 ± 0.18 c |
| FW 20 min | 100.06 ± 0.01 c | 0.23 ± 0.02 bc | −0.04 ± 0.03 c | 0.24 ± 0.02 c |
| FW 30 min | 100.08 ± 0.01 bc | 0.06 ± 0.07 d | 0.32 ± 0.08 c | 0.34 ± 0.07 c |
| L* | a* | b* | ΔE | |
|---|---|---|---|---|
| AF 0 min | 100.14 ± 0.01 a | 0.42 ± 0.02 c | 10.78 ± 0.08 fg | 10.79 ± 0.08 fg |
| AF 10 min | 100.05 ± 0.01 b | −3.77 ± 0.02 e | 7.57 ± 0.03 gh | 8.46 ± 0.03 gh |
| AF 20 min | 95.72 ± 0.01 h | −14.44 ± 0.02 k | 68.61 ± 13.31 d | 70.30 ± 13.00 d |
| AF 30 min | 62.54 ± 0.03 j | 20.82 ± 0.03 a | 99.35 ± 0.07 a | 108.20 ± 0.06 a |
| C 0 min | 100.08 ± 0.01 b | −1.19 ± 0.04 d | 2.52 ± 0.07 hi | 2.79 ± 0.08 h |
| C 10 min | 99.81 ± 0.00 d | −5.82 ± 0.02 g | 9.22 ± 0.06 fg | 10.90 ± 0.06 fg |
| C 20 min | 99.79 ± 0.12 d | −8.03 ± 0.05 i | 14.27 ± 0.09 f | 16.38 ± 0.10 f |
| C 30 min | 99.03 ± 0.01 f | −12.47 ± 0.03 j | 25.71 ± 0.05 e | 28.59 ± 0.03 e |
| AFC 0 min | 99.66 ± 0.04 e | −7.16 ± 0.06 h | −0.16 ± 0.03 i | 7.17 ± 0.06 gh |
| AFC 10 min | 99.98 ± 0.01 c | −4.18 ± 0.02 f | 7.52 ± 0.03 gh | 8.60 ± 0.03 gh |
| AFC 20 min | 95.86 ± 0.06 g | −14.36 ± 0.47 k | 83.89 ± 0.02 c | 85.21 ± 0.06 cb |
| AFC 30 min | 75.21 ± 0.04 i | 14.37 ± 0.03 b | 91.86 ± 0.09 b | 96.23 ± 0.07 cb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-T.V.; Ting, Y.-S.; Ndraha, N.; Hsiao, H.-I.; Sung, W.-C. Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants. Polymers 2022, 14, 1565. https://doi.org/10.3390/polym14081565
Lin H-TV, Ting Y-S, Ndraha N, Hsiao H-I, Sung W-C. Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants. Polymers. 2022; 14(8):1565. https://doi.org/10.3390/polym14081565
Chicago/Turabian StyleLin, Hong-Ting Victor, Yen-Shu Ting, Nodali Ndraha, Hsin-I Hsiao, and Wen-Chieh Sung. 2022. "Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants" Polymers 14, no. 8: 1565. https://doi.org/10.3390/polym14081565
APA StyleLin, H. -T. V., Ting, Y. -S., Ndraha, N., Hsiao, H. -I., & Sung, W. -C. (2022). Effect of Chitosan Incorporation on the Development of Acrylamide during Maillard Reaction in Fructose–Asparagine Model Solution and the Functional Characteristics of the Resultants. Polymers, 14(8), 1565. https://doi.org/10.3390/polym14081565