Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications
Abstract
:1. Introduction
2. Preparation of Elastomer–Hydrogel Systems
3. Bio-Inspiration for Strong Interface Formation between Elastomers and Hydrogels
4. Components of Elastomer–Hydrogel Systems by Their Origin
4.1. Elastomers
4.1.1. Natural Elastomers
4.1.2. Synthetic Elastomers
4.2. Hydrogels
4.2.1. Natural Hydrogels
4.2.2. Synthetic and Semisynthetic Hydrogels
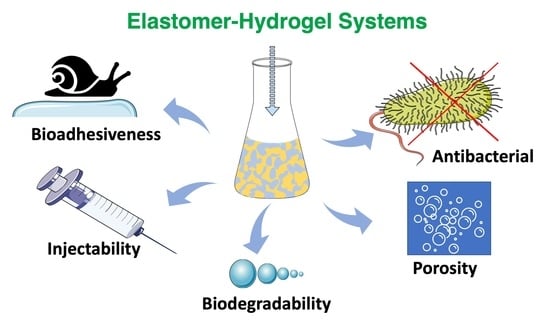
5. Biofunctionalities of Elastomer–Hydrogel Systems
5.1. Bioadhesiveness
5.2. Injectability
5.3. Biodegradation
5.4. Porosity
5.5. Antibacterial Surfaces
6. Elastomer–Hydrogel Systems for Soft Tissue Engineering Applications
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J.Y.H. 3D Bioprinting of Tissues and Organs for Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Teramura, Y.; Ekdahl, K.N.; Barbu, A. A Hybrid of Cells and Pancreatic Islets toward a New Bioartificial Pancreas. Regen. Ther. 2016, 3, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Weiss, D.E.; Liu, Q.; Tian, B. Biomimetic Approaches toward Smart Bio-Hybrid Systems. Nano Res. 2018, 11, 3009–3030. [Google Scholar] [CrossRef]
- Walia, R.; Akhavan, B.; Kosobrodova, E.; Kondyurin, A.; Oveissi, F.; Naficy, S.; Yeo, G.C.; Hawker, M.; Kaplan, D.L.; Dehghani, F.; et al. Hydrogel−Solid Hybrid Materials for Biomedical Applications Enabled by Surface-Embedded Radicals. Adv. Funct. Mater. 2020, 30, 2004599. [Google Scholar] [CrossRef]
- Ozdil, D.; Aydin, H.M. Polymers for Medical and Tissue Engineering Applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Li, J.; Osada, Y.; Cooper-White, J. (Eds.) Functional Hydrogels as Biomaterials; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018; Volume 12, ISBN 978-3-662-57509-3. [Google Scholar]
- Król, P. Synthesis Methods, Chemical Structures and Phase Structures of Linear Polyurethanes. Properties and Applications of Linear Polyurethanes in Polyurethane Elastomers, Copolymers and Ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Amsden, B. Curable, Biodegradable Elastomers: Emerging Biomaterials for Drug Delivery and Tissue Engineering. Soft Matter 2007, 3, 1335–1348. [Google Scholar] [CrossRef]
- Hassouna, Y.M.; Zamani, S.; Kafienah, W.; Younes, H.M. Synthesis, Characterization & Cytocompatibility of Poly (Diol-Co-Tricarballylate) Based Thermally Crosslinked Elastomers for Drug Delivery & Tissue Engineering Applications. Mater. Sci. Eng. C 2018, 93, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Opris, D.M. Polar Elastomers as Novel Materials for Electromechanical Actuator Applications. Adv. Mater. 2018, 30, 1–23. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Yepez, G.; Wei, Z.; Liu, F.; Bugarin, A.; Tang, L.; Hong, Y. Development of Dopant-Free Conductive Bioelastomers. Sci. Rep. 2016, 6, 34451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer Artificial Muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Jeong, K.H.; Kim, J.; Lee, L.P. Biologically Inspired Artificial Compound Eyes. Science 2006, 312, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Christman, K.L.; Vardanian, A.J.; Fang, Q.; Sievers, R.E.; Fok, H.H.; Lee, R.J. Injectable Fibrin Scaffold Improves Cell Transplant Survival, Reduces Infarct Expansion, and Induces Neovasculature Formation in Ischemic Myocardium. J. Am. Coll. Cardiol. 2004, 44, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Harding, S.E.; Ali, N.N.; Lyon, A.R.; Boccaccini, A.R. Biomaterials in Cardiac Tissue Engineering: Ten Years of Research Survey. Mater. Sci. Eng. R Rep. 2008, 59, 1–37. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Djordjevic, I.; Pingguan-Murphy, B.; Kadri, N.A.; Towler, M.R. Bioactive Glass Reinforced Elastomer Composites for Skeletal Regeneration: A Review. Mater. Sci. Eng. C 2015, 53, 175–188. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric Biomaterials for Tissue Engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Tian, Y.; Liang, K.; Wang, X.; Ji, Y. Fabrication of Nanocomposite Bioelastomer Porous Scaffold Based on Chitin Nanocrystal Supported Emulsion-Freeze-Casting. ACS Sustain. Chem. Eng. 2017, 5, 3305–3313. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated Hydrogels for Tissue Engineering and Regenerative Medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9, 1088–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Xu, C.; Okpokwasili, C.; Huang, Y.; Shi, X.; Wu, J.; Liao, J.; Tang, L.; Hong, Y. Optimizing Anisotropic Polyurethane Scaffolds to Mechanically Match with Native Myocardium. ACS Biomater. Sci. Eng. 2020, 6, 2757–2769. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the Biomimetic Behavior of Scaffolds for Regenerative Medicine through Surface Modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Olbrich, J.M.; Tate, P.L.; Corbett, J.T.; Lindsey, J.M.; Nagatomi, S.D.; Shalaby, W.S.W.; Shalaby, S.W. Injectable in Situ Forming Controlled Release Implant Composed of a Poly-Ether-Ester-Carbonate and Applications in the Field of Chemotherapy. J. Biomed. Mater. Res. Part A 2012, 100, 2365–2372. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773. [Google Scholar] [CrossRef] [Green Version]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Y.S.; Bakht, S.M.; Aleman, J.; Shin, S.R.; Yue, K.; Sica, M.; Ribas, J.; Duchamp, M.; Ju, J.; et al. Elastomeric Free-Form Blood Vessels for Interconnecting Organs on Chip Systems. Lab Chip 2016, 16, 1579–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, M.E.; Mellert, L.T.; Firstenberg, M.S. Bedside Procedure: Retained Central Venous Catheter. In Vignettes in Patient Safety; InTech: London, UK, 2018; Volume 2. [Google Scholar]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive Anti-Oxidant Polyurethane Elastomers with Shape Memory Property as Non-Adherent Wound Dressing to Enhance Wound Healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic-Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Shi, R.; Chen, D.; Liu, Q.; Wu, Y.; Xu, X.; Zhang, L.; Tian, W. Recent Advances in Synthetic Bioelastomers. Int. J. Mol. Sci. 2009, 10, 4223–4256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thakur, V.K.; Li, Y.; Garrison, T.F.; Gao, Z.; Gu, J.; Kessler, M.R. Soybean-Oil-Based Thermosetting Resins with Methacrylated Vanillyl Alcohol as Bio-Based, Low-Viscosity Comonomer. Macromol. Mater. Eng. 2018, 303, 1700278. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Nian, G.; Yang, C.; Qu, S.; Suo, Z. Bonding Dissimilar Polymer Networks in Various Manufacturing Processes. Nat. Commun. 2018, 9, 846. [Google Scholar] [CrossRef]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant Tough Bonding of Hydrogels for Soft Machines and Electronics. Sci. Adv. 2017, 3, 1700053. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-Inspired Hydrogel–Elastomer Hybrids with Robust Interfaces and Functional Microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [Green Version]
- North, M.A.; Del Grosso, C.A.; Wilker, J.J. High Strength Underwater Bonding with Polymer Mimics of Mussel Adhesive Proteins. ACS Appl. Mater. Interfaces 2017, 9, 7866–7872. [Google Scholar] [CrossRef]
- Nishida, J.; Higaki, Y.; Takahara, A. Synthesis and Characterization of Barnacle Adhesive Mimetic towards Underwater Adhesion. Chem. Lett. 2015, 44, 1047–1049. [Google Scholar] [CrossRef] [Green Version]
- Glass, P.; Chung, H.; Washburn, N.R.; Sitti, M. Enhanced Reversible Adhesion of Dopamine Methacrylamide-Coated Elastomer Microfibrillar Structures under Wet Conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence Basis of Barnacle Cement Nanostructure Is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6, 36219. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Tanzer, M.L. Polyphenolic Substance of Mytilus Edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Lee, Y.; Le Thi, P.; Park, K.D. Engineered Horseradish Peroxidase-Catalyzed Hydrogels with High Tissue Adhesiveness for Biomedical Applications. J. Ind. Eng. Chem. 2019, 78, 34–52. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A Reversible Wet/Dry Adhesive Inspired by Mussels and Geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.D.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E.; et al. A Biodegradable and Biocompatible Gecko-Inspired Tissue Adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Aghaei-Ghareh-Bolagh, B.; Mithieux, S.M.; Weiss, A.S. Elastic Proteins and Elastomeric Protein Alloys. Curr. Opin. Biotechnol. 2016, 39, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Mahara, A.; Tong, Z.; Levenson, E.A.; Mcgann, C.L.; Jia, X.; Yamaoka, T.; Kiick, K.L. Recombinant Resilin-Based Bioelastomers for Regenerative Medicine Applications. Adv. Healthc. Mater. 2016, 5, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Y.; McKittrick, J.; Meyers, M.A. Biological Materials: Functional Adaptations and Bioinspired Designs. Prog. Mater. Sci. 2012, 57, 1492–1704. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Fibrin Mechanical Properties and Their Structural Origins. Matrix Biol. 2017, 60, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Zhmurov, A.; Brown, A.E.X.; Litvinov, R.I.; Dima, R.I.; Weisel, J.W.; Barsegov, V. Mechanism of Fibrin(Ogen) Forced Unfolding. Structure 2011, 19, 1615–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodabandeh, Z.; Tanideh, N.; Aslani, F.S.; Jamhiri, I.; Zare, S.; Alizadeh, N.; Safari, A.; Farshidfar, N.; Dara, M.; Zarei, M. A Comparative in Vitro and in Vivo Study on Bone Tissue Engineering Potential of the Collagen/Nano-Hydroxyapatite Scaffolds Loaded with Ginger Extract and Curcumin. Mater. Today Commun. 2022, 31, 103339. [Google Scholar] [CrossRef]
- Li, L.; Charati, M.B.; Kiick, K.L. Elastomeric Polypeptide-Based Biomaterials. Polym. Chem. 2010, 1, 1160–1170. [Google Scholar] [CrossRef] [Green Version]
- Jennewein, C.; Tran, N.; Paulus, P.; Ellinghaus, P.; Eble, J.A.; Zacharowski, K. Novel Aspects of Fibrin(Ogen) Fragments during Inflammation. Mol. Med. 2011, 17, 568–573. [Google Scholar] [CrossRef]
- Hejbøl, E.K.; Sellathurai, J.; Nair, P.D.; Schrøder, H.D. Injectable Scaffold Materials Differ in Their Cell Instructive Effects on Primary Human Myoblasts. J. Tissue Eng. 2017, 8, 2041731417717677. [Google Scholar] [CrossRef] [Green Version]
- Vendamme, R.; Eevers, W. Sticky Degradable Bioelastomers. Chem. Mater. 2017, 29, 5353–5363. [Google Scholar] [CrossRef]
- Puskas, J.E.; Chen, Y. Biomedical Application of Commercial Polymyers and Novel Polyisobutylene-Based Thermoplastic Elastomers for Soft Tissue Replacement. Biomacromolecules 2004, 5, 1141–1154. [Google Scholar] [CrossRef]
- Vogt, L.; Rivera, L.R.; Liverani, L.; Piegat, A.; El Fray, M.; Boccaccini, A.R. Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) Electrospun Scaffolds for Cardiac Tissue Engineering Using Benign Solvents. Mater. Sci. Eng. C 2019, 103, 109712. [Google Scholar] [CrossRef]
- Izraylit, V.; Gould, O.E.C.; Rudolph, T.; Kratz, K.; Lendlein, A. Controlling Actuation Performance in Physically Cross-Linked Polylactone Blends Using Polylactide Stereocomplexation. Biomacromolecules 2020, 21, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Li, X.; Wang, Y.; Fan, L.J.; Yang, S.; Guo, M.; Li, X.; Tu, Y. Supramolecular and Physically Double-Cross-Linked Network Strategy toward Strong and Tough Elastic Fibers. ACS Macro Lett. 2020, 9, 1655–1661. [Google Scholar] [CrossRef]
- Martinez, H.; Hillmyer, M.A. Carboxy-Telechelic Polyolefins in Cross-Linked Elastomers. Macromolecules 2014, 47, 479–485. [Google Scholar] [CrossRef]
- Amsden, B.; Wang, S.; Wyss, U. Synthesis and Characterization of Thermoset Biodegradable Elastomers Based on Star-Poly (ε-Caprolactone-Co-D,L-Lactide). Biomacromolecules 2004, 5, 1399–1403. [Google Scholar] [CrossRef]
- Puskas, J.E.; Foreman-Orlowski, E.A.; Lim, G.T.; Porosky, S.E.; Evancho-Chapman, M.M.; Schmidt, S.P.; El Fray, M.; Piatek, M.; Prowans, P.; Lovejoy, K. A Nanostructured Carbon-Reinforced Polyisobutylene-Based Thermoplastic Elastomer. Biomaterials 2010, 31, 2477–2488. [Google Scholar] [CrossRef]
- Hodge, P.; O’Dell, R.; Lee, M.S.K.; Ebdon, J.R. Synthesis of Polyesters by Reaction of Carboxylic Acid Quaternary Ammonium Salts with Alkyl Halides or Alkyl Tosylates. Polymer 1996, 37, 1267–1271. [Google Scholar] [CrossRef]
- Wcislek, A.; Olalla, A.S.; McClain, A.; Piegat, A.; Sobolewski, P.; Puskas, J.; El Fray, M. Enzymatic Degradation of Poly(Butylene Succinate) Copolyesters Synthesized with the Use of Candida Antarctica Lipase B. Polymers 2018, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Liverani, L.; Piegat, A.; Niemczyk, A.; El Fray, M.; Boccaccini, A.R. Electrospun Fibers of Poly(Butylene Succinate–Co–Dilinoleic Succinate) and Its Blend with Poly(Glycerol Sebacate) for Soft Tissue Engineering Applications. Eur. Polym. J. 2016, 81, 295–306. [Google Scholar] [CrossRef]
- Sonseca, A.; Sahay, R.; Stepien, K.; Bukala, J.; Wcislek, A.; McClain, A.; Sobolewski, P.; Sui, X.; Puskas, J.E.; Kohn, J.; et al. Architectured Helically Coiled Scaffolds from Elastomeric Poly(Butylene Succinate) (PBS) Copolyester via Wet Electrospinning. Mater. Sci. Eng. C 2020, 108, 110505. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Liang, K.; Ji, Y. Degradable Bioelastomers Prepared by a Facile Melt Polycondensation of Citric Acid and Polycaprolactone-Diol. J. Macromol. Sci. Part B Phys. 2018, 57, 679–690. [Google Scholar] [CrossRef]
- Guo, J.; Nguyen, D.Y.; Tran, R.T.; Xie, Z.; Bai, X.; Yang, J. Design Strategies and Applications of Citrate-Based Biodegradable Elastomeric Polymers. Nat. Synth. Biomed. Polym. 2014, 1, 259–285. [Google Scholar] [CrossRef]
- Nijst, C.L.E.; Bruggeman, J.P.; Karp, J.M.; Ferreira, L.; Zumbuehl, A.; Bettinger, C.J.; Langer, R. Synthesis and Characterization of Photocurable Elastomers from Poly(Glycerol-Co-Sebacate). Biomacromolecules 2007, 8, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, Y.M.; Langer, R. In Vivo Degradation Characteristics of Poly(Glycerol Sebacate). J. Biomed. Mater. Res. Part A 2003, 66, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Liverani, L.; Roether, J.A.; Boccaccini, A.R. Electrospun Zein Fibers Incorporating Poly(Glycerol Sebacate) for Soft Tissue Engineering. Nanomaterials 2018, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Mitsak, A.G.; Dunn, A.M.; Hollister, S.J. Mechanical Characterization and Non-Linear Elastic Modeling of Poly(Glycerol Sebacate) for Soft Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 3–15. [Google Scholar] [CrossRef]
- Dippold, D.; Tallawi, M.; Tansaz, S.; Roether, J.A.; Boccaccini, A.R. Novel Electrospun Poly(Glycerol Sebacate)-Zein Fiber Mats as Candidate Materials for Cardiac Tissue Engineering. Eur. Polym. J. 2016, 75, 504–513. [Google Scholar] [CrossRef]
- Tallá Ferrer, C.; Vilariño-Feltrer, G.; Rizk, M.; Sydow, H.G.; Vallés-Lluch, A. Nanocomposites Based on Poly(Glycerol Sebacate) with Silica Nanoparticles with Potential Application in Dental Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 761–772. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef]
- Kanemura, C.; Nakashima, S.; Hotta, A. Mechanical Properties and Chemical Structures of Biodegradable Poly(Butylene-Succinate) for Material Reprocessing. Polym. Degrad. Stab. 2012, 97, 972–980. [Google Scholar] [CrossRef]
- Hassan, E.; Wei, Y.; Jiao, H.; Muhuo, Y. Dynamic Mechanical Properties and Thermal Stability of Poly(Lactic Acid) and Poly(Butylene Succinate) Blends Composites. J. Fiber Bioeng. Inform. 2013, 6, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Yu, J.; Cheng, L.; Qu, W. Mechanical Properties of Poly(Butylene Succinate) (PBS) Biocomposites Reinforced with Surface Modified Jute Fibre. Compos. Part A Appl. Sci. Manuf. 2009, 40, 669–674. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Ickowicz, D.E.; Haim-Zada, M.; Abbas, R.; Touitou, D.; Nyska, A.; Golovanevski, L.; Weiniger, C.F.; Katzhendler, J.; Domb, A.J. Castor Oil-Citric Acid Copolyester for Tissue Augmentation. Polym. Adv. Technol. 2014, 25, 1323–1328. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Ishii, O.; Sueda, T.; Vacanti, J.P. Contractile Cardiac Grafts Using a Novel Nanofibrous Mesh. Biomaterials 2004, 25, 3717–3723. [Google Scholar] [CrossRef]
- Tateishi, T.; Chen, G.P. Biodegradable Polymer Scaffold for Tissue Engineering. Key Eng. Mater. 2005, 288–289, 59–62. [Google Scholar] [CrossRef]
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun Poly(Butylene Succinate)/Cellulose Nanocrystals Bio-Nanocomposite Scaffolds for Tissue Engineering: Preparation, Characterization and In Vitro Evaluation. Polym. Test. 2018, 71, 101–109. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent Progress in Polymeric Non-Invasive Insulin Delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef]
- Wu, C.; Long, L.; Zhang, Y.; Xu, Y.; Lu, Y.; Yang, Z.; Guo, Y.; Zhang, J.; Hu, X.; Wang, Y. Injectable Conductive and Angiogenic Hydrogels for Chronic Diabetic Wound Treatment. J. Control. Release 2022, 344, 249–260. [Google Scholar] [CrossRef]
- Wei, C.; Jin, X.; Wu, C.; Zhang, W. Injectable Composite Hydrogel Based on Carbon Particles for Photothermal Therapy of Bone Tumor and Bone Regeneration. J. Mater. Sci. Technol. 2022, 118, 64–72. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Liu, J.; Wang, X.; Guo, H.; Xue, L.; Li, L.; Li, J.; Zhang, B.; Xue, Y.; et al. An Enzyme Cross-Linked Hydrogel as a Minimally Invasive Arterial Tissue Sealing and Anti-Adhesion Barrier. Nano Today 2022, 44, 101467. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural Hydrogels for Cartilage Regeneration: Modification, Preparation and Application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable Natural Polymer Hydrogels for Articular Cartilage Tissue Engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable Chitosan Hydrogels for Localised Cancer Therapy. J. Control. Release 2008, 126, 205–216. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.; Mo, X.; Pan, J. In Situ Forming Hydrogel of Natural Polysaccharides through Schiff Base Reaction for Soft Tissue Adhesive and Hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef]
- Gholamali, I. Stimuli-Responsive Polysaccharide Hydrogels for Biomedical Applications: A Review. Regen. Eng. Transl. Med. 2019, 7, 91–114. [Google Scholar] [CrossRef]
- Gun’ko, V.; Savina, I.; Mikhalovsky, S. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Park, S.H.; Seo, J.Y.; Park, J.Y.; Ji, Y.B.; Kim, K.; Choi, H.S.; Choi, S.; Kim, J.H.; Min, B.H.; Kim, M.S. An Injectable, Click-Crosslinked, Cytomodulin-Modified Hyaluronic Acid Hydrogel for Cartilage Tissue Engineering. NPG Asia Mater. 2019, 11, 30. [Google Scholar] [CrossRef]
- He, W.; Reaume, M.; Hennenfent, M.; Lee, B.P.; Rajachar, R. Biomimetic Hydrogels with Spatial- and Temporal-Controlled Chemical Cues for Tissue Engineering. Biomater. Sci. 2020, 8, 3248–3269. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Van Bochove, B.; Grijpma, D.W. Photo-Crosslinked Synthetic Biodegradable Polymer Networks for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef] [Green Version]
- Mirtaghavi, A.; Baldwin, A.; Tanideh, N.; Zarei, M.; Muthuraj, R.; Cao, Y.; Zhao, G.; Geng, J.; Jin, H.; Luo, J. Crosslinked Porous Three-Dimensional Cellulose Nanofibers-Gelatine Biocomposite Scaffolds for Tissue Regeneration. Int. J. Biol. Macromol. 2020, 164, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Zant, E.; Grijpma, D.W. Synthetic Biodegradable Hydrogels with Excellent Mechanical Properties and Good Cell Adhesion Characteristics Obtained by the Combinatorial Synthesis of Photo-Cross-Linked Networks. Biomacromolecules 2016, 17, 1582–1592. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Physical and Chemical Characterisation of Acrylamide-Based Hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Mahmoudi Khatir, N. Investigation of Acyclovir-Loaded, Acrylamide-Based Hydrogels for Potential Use as Vaginal Ring. Mater. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]
- Lee, J.; Silberstein, M.N.; Abdeen, A.A.; Kim, S.Y.; Kilian, K.A. Mechanochemical Functionalization of Disulfide Linked Hydrogels. Mater. Horizons 2016, 3, 447–451. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Gull, N.; Khan, S.M.; Munawar, M.A.; Shafiq, M.; Sabir, A.; Jamil, T. Evaluation of Selected Properties of Biocompatible Chitosan/Poly(Vinyl Alcohol) Blends. Int. J. Biol. Macromol. 2016, 82, 551–556. [Google Scholar] [CrossRef]
- Hu, D.; Cui, Y.; Mo, K.; Wang, J.; Huang, Y.; Miao, X.; Lin, J.; Chang, C. Ultrahigh Strength Nanocomposite Hydrogels Designed by Locking Oriented Tunicate Cellulose Nanocrystals in Polymeric Networks. Compos. Part B Eng. 2020, 197, 108118. [Google Scholar] [CrossRef]
- Zhu, L.; Qiu, J.; Sakai, E. A High Modulus Hydrogel Obtained from Hydrogen Bond Reconstruction and Its Application in Vibration Damper. RSC Adv. 2017, 7, 43755–43763. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Qiu, J.; Sakai, E.; Zang, L.; Yu, Y.; Ito, K.; Liu, P.; Kang, F. Design of a Rubbery Carboxymethyl Cellulose/Polyacrylic Acid Hydrogel via Visible-Light-Triggered Polymerization. Macromol. Mater. Eng. 2017, 302, 1–9. [Google Scholar] [CrossRef]
- Przeradzka, M.A.; van Bochove, B.; Bor, T.C.; Grijpma, D.W. Phase-Separated Mixed-Macromer Hydrogel Networks and Scaffolds Prepared by Stereolithography. Polym. Adv. Technol. 2017, 28, 1212–1218. [Google Scholar] [CrossRef]
- Reece, T.B.; Maxey, T.S.; Kron, I.L. A Prospectus on Tissue Adhesives. Am. J. Surg. 2001, 182, S40–S44. [Google Scholar] [CrossRef]
- Chen, Y.M.; Ogawa, R.; Kakugo, A.; Osada, Y.; Gong, J.P. Dynamic Cell Behavior on Synthetic Hydrogels with Different Charge Densities. Soft Matter 2009, 5, 1804. [Google Scholar] [CrossRef]
- Berkovitch, Y.; Seliktar, D. Semi-Synthetic Hydrogel Composition and Stiffness Regulate Neuronal Morphogenesis. Int. J. Pharm. 2017, 523, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; De Manzini, N.; Paoletti, S. Adhesive and Sealant Interfaces for General Surgery Applications. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wu, L.; Yan, H.; Qu, L.; Wang, L.; Wang, X.; Ren, S.; Kong, D.; Wang, L. Multifunctional Hydrogel Patch with Toughness, Tissue Adhesiveness, and Antibacterial Activity for Sutureless Wound Closure. ACS Biomater. Sci. Eng. 2019, 5, 2610–2620. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Villalobos-García, R.; Alvarez-Colín, E.; Cornejo-Bravo, J.M. In Vitro Evaluation of the Bioadhesive Properties of Hydrophobic Polybasic Gels Containing N,N-Dimethylaminoethyl Methacrylate-Co-Methyl Methacrylate. Biomaterials 2001, 22, 957–961. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Yu, C.H.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a Naturally-Derived Adhesive and Conductive Cardiopatch. Biomaterials 2019, 207, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The Chemistry of Tissue Adhesive Materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Tian, K.; Bae, J.; Suo, Z.; Vlassak, J.J. Adhesion between Hydrophobic Elastomer and Hydrogel through Hydrophilic Modification and Interfacial Segregation. ACS Appl. Mater. Interfaces 2018, 10, 43252–43261. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.J.; Suo, Z. Printing Hydrogels and Elastomers in Arbitrary Sequence with Strong Adhesion. Adv. Funct. Mater. 2019, 29, 1901721. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H. Adhesion a La Moule. Biochemistry 2002, 1180, 1172–1180. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Neumeyer, J.L.; Baldessarini, R.J.; Zhen, X.; Zhang, A. Update 1 of: Recent Progress in Development of Dopamine Receptor Subtype-Selective Agents: Potential Therapeutics for Neurological and Psychiatric Disorders. Chem. Rev. 2013, 113, 274–302. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Vázquez-Lasa, B.; San Román, J. Bioactive and Bioadhesive Catechol Conjugated Polymers for Tissue Regeneration. Polymers 2018, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for PH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
- Patenaude, M.; Smeets, N.M.B.; Hoare, T. Designing Injectable, Covalently Cross-Linked Hydrogels for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu Priya, M.; Nair, S.V.; Jayakumar, R. An Overview of Injectable Polymeric Hydrogels for Tissue Engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Li, C.; Huang, Z.; Gao, N.; Zheng, J.; Guan, J. Injectable, Thermosensitive, Fast Gelation, Bioeliminable, and Oxygen Sensitive Hydrogels. Mater. Sci. Eng. C 2019, 99, 1191–1198. [Google Scholar] [CrossRef]
- Vo, T.N.; Shah, S.R.; Lu, S.; Tatara, A.M.; Lee, E.J.; Roh, T.T.; Tabata, Y.; Mikos, A.G. Injectable Dual-Gelling Cell-Laden Composite Hydrogels for Bone Tissue Engineering. Biomaterials 2016, 83, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Wu, Y.; Gu, Q.-S.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Injectable Photo Crosslinked Enhanced Double-Network Hydrogels from Modified Sodium Alginate and Gelatin. Int. J. Biol. Macromol. 2017, 96, 569–577. [Google Scholar] [CrossRef]
- Xu, Q.; Sigen, A.; Gao, Y.; Guo, L.; Creagh-Flynn, J.; Zhou, D.; Greiser, U.; Dong, Y.; Wang, F.; Tai, H.; et al. A Hybrid Injectable Hydrogel from Hyperbranched PEG Macromer as a Stem Cell Delivery and Retention Platform for Diabetic Wound Healing. Acta Biomater. 2018, 75, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Skrobot, J.; Zair, L.; Ostrowski, M.; El Fray, M. New Injectable Elastomeric Biomaterials for Hernia Repair and Their Biocompatibility. Biomaterials 2016, 75, 182–192. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L. Synthesis, Preparation, In Vitro Degradation, and Application of Novel Degradable Bioelastomers—A Review. Prog. Polym. Sci. 2012, 37, 715–765. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(Butylene Succinate)-Based Polyesters for Biomedical Applications: A Review in Memory of Our Beloved Colleague and Friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Piarali, S.; Marlinghaus, L.; Viebahn, R.; Lewis, H.; Ryadnov, M.G.; Groll, J.; Salber, J.; Roy, I. Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 8, 442. [Google Scholar] [CrossRef]
- Skrobot, J.; Ignaczak, W.; El Fray, M. Hydrolytic and Enzymatic Degradation of Fl Exible Polymer Networks Comprising Fatty Acid Derivatives. Polym. Degrad. Stab. 2015, 120, 368–376. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Santerre, J.P.; Labow, R.S.; Duguay, D.G.; Erfle, D.; Adams, G.A. Biodegradation Evaluation of Polyether and Polyester-Urethanes with Oxidative and Hydrolytic Enzymes. J. Biomed. Mater. Res. 1994, 28, 1187–1199. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, B.B.; Kundu, S.C. Cell Proliferation and Migration in Silk Fibroin 3D Scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of Pore Size on ECM Secretion and Cell Growth in Gelatin Scaffold for Articular Cartilage Tissue Engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan Scaffolds: Interconnective Pore Size and Cartilage Engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of Macroporous Protein Scaffolds on Bone Tissue Engineering from Bone Marrow Stem Cells. Biomaterials 2005, 26, 4442–4452. [Google Scholar] [CrossRef]
- Dutta Roy, T.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of Degradable Composite Bone Repair Products Made via Three-Dimensional Fabrication Techniques. J. Biomed. Mater. Res. Part A 2003, 66, 283–291. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S.; Kaviyarasu, K. Development of Biomimetic Hybrid Porous Scaffold of Chitosan/Polyvinyl Alcohol/Carboxymethyl Cellulose by Freeze-Dried and Salt Leached Technique. J. Nanosci. Nanotechnol. 2017, 18, 4916–4922. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef]
- Muzammil, K.M.; Mukherjee, D.; Azamthulla, M.; Teja, B.V.; Kaamnoore, D.; Anbu, J.; Srinivasan, B.; Jeevan Kasture, G. Castor Oil Reinforced Polymer Hybrids for Skin Tissue Augmentation. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 530–544. [Google Scholar] [CrossRef]
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic Hybrid Systems for Tissue Engineering. Biomimetics 2020, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.; Ahumada, M.; Andronic, C.; McNeill, B.; Variola, F.; Griffith, M.; Ruel, M.; Hamel, V.; Liang, W.; Suuronen, E.J.; et al. Electroconductive Nanoengineered Biomimetic Hybrid Fibers for Cardiac Tissue Engineering. J. Mater. Chem. B 2017, 5, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-Kafrani, A.; Samadikuchaksaraei, A.; Kaplan, D.L.; Moroni, L.; Joghataei, M.T. Hybrid and Composite Scaffolds Based on Extracellular Matrices for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2019, 25, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Neves, S.C.; Moroni, L.; Barrias, C.C.; Granja, P.L. Leveling Up Hydrogels: Hybrid Systems in Tissue Engineering. Trends Biotechnol. 2020, 38, 292–315. [Google Scholar] [CrossRef]
- Fischenich, K.M.; Lewis, J.T.; Bailey, T.S.; Haut Donahue, T.L. Mechanical Viability of a Thermoplastic Elastomer Hydrogel as a Soft Tissue Replacement Material. J. Mech. Behav. Biomed. Mater. 2018, 79, 341–347. [Google Scholar] [CrossRef]
- Lewis, J.T.; Fischenich, K.M.; Haut Donahue, T.L.; Bailey, T.S. Nanostructure-Driven Replication of Soft Tissue Biomechanics in a Thermoplastic Elastomer Hydrogel. ACS Biomater. Sci. Eng. 2018, 4, 3854–3863. [Google Scholar] [CrossRef]
- Remya, K.R.; Chandran, S.; Mani, S.; John, A.; Ramesh, P. Hybrid Polycaprolactone/Polyethylene Oxide Scaffolds with Tunable Fiber Surface Morphology, Improved Hydrophilicity and Biodegradability for Bone Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1444–1462. [Google Scholar] [CrossRef]












| Proteins | Dominant Amino Acids | Distribution | Young Modulus [Mpa] | Tensile Strength [Mpa] | Elongation [%] | References |
|---|---|---|---|---|---|---|
| Collagen | 35% Glycine, 12% Proline | Bone, teeth, vasculature, organs | 100–2900 | 5–500 | 5–50 | [53] |
| Elastin | 32% Glycine, 21% Alanine | Skin, lungs, vasculature | 0.3–0.6 | 0.36–4.4 | 100–220 | [15] |
| Fibrin | 45% Glycine, 30% Alanine | Blood | 1.7–14.5 | 0.01–0.02 | 100 | [54,55] |
| Material | Crosslinking Type | Young Modulus [Mpa] | Tensile Strength [Mpa] | Elongation [%] | References |
|---|---|---|---|---|---|
| Poly(diol citrate) (PCC) | chemical | 140–1737 * | 171–977 * | 70–260 | [72] |
| Poly(glycerol sebacate) (PGS) | chemical | 0.056–1.5 | 0.5 | 40–450 | [77] |
| Dopant-free conductive polyurethane elastomer (DCPU) | chemical | 0.5–3.8 | 9.6–20.3 | 170–190 | [26] |
| Poly(glycerol sebacate-co-acrylate) (PGSA) | chemical | 0.05–1.38 | 0.05–0.5 | 42–189 | [74] |
| Poly(caprolactone) (PCL) | physical | 210–340 | 10.0–60.0 | 300–1200 | [80,81,82] |
| Poly(butylene succinate) (PBS) | physical | 550 | 20.0–40.7 | 100–224 | [83,84,85] |
| Poly(glycolic acid) (PGA) | physical | 6900 | 68.9 | 15–20 | [80] |
| Elastomer | Advantages | Disadvantages | References |
|---|---|---|---|
| Collagen | Wide range of elasticity depending on the origin of protein, can be prepared by crosslinking, show low antigenicity | hard to control degradability | [57] |
| Elastin | Rubber-like properties | demanding purification process, high propensity to calcification | [15,31] |
| Fibrin | Bioactivity (mitogenic, chemotactic and proangiogenic activities), degradation products (coagulation and fibrinolysis) are activators of wound repair | rapid degradation | [54] |
| Poly(lactic acid) (PLA) | Easy to print (low melting point), highly biocompatible and biodegradable | the lack of cell-recognition signals | [88] |
| Poly(glycerol sebacate) (PGS) | Can mimic mechanical properties of collagen and elastin, degradation product are a natural metabolic compound | the lack of cell-recognition signals | [74,77] |
| Poly(ε-caprolactone) (PCL) | Highly elastic, slow degradation time (1–2 years) | the lack of cell-recognition signals | [89,90] |
| Poly(butylene succinate) (PBS) | Controlled biodegradability, | the lack of cell-recognition signals | [91] |
| Synthetic HGs | Crosslinking Type | Young Modulus [Mpa] | Tensile Strength [Mpa] | Elongation [%] | References |
|---|---|---|---|---|---|
| Poly(ethylene glycol) (PEG)/polydimethylsiloxane (PDMS) hydrogel | chemical | 0.006–0.36 | 0.02–0.42 | 30 | [112] |
| Chitosan (CS) and poly(vinyl alcohol) (PVA) (CS/PVA) | chemical | 2.3–2.5 | 6.0–9.70 | 16.3–28.1 | [113] |
| Tunicate cellulose nanocrystals (TCNCs) aligned (anisotropic d-Gel) | physical | 152.1 | 13.7–56.2 | 1400 | [114] |
| Aluminum ion cross-linked hydrogel (Gel) high-modulus hydrogels (HM-Gel) | physical | 0.59–1.94 | 1.26–1.74 | 550–650 | [115] |
| Carboxymethyl cellulose/polyacrylic acid hydrogel (CMC/PAA) | physical | 0.065–0.18 | 0.40–0.85 | 350–700 | [116] |
| PDLLA-dMA-PCL-dMA-PEG-dMA hydrogel | physical | 1.4 ± 0.2 | 0.47 ± 0.06 | 84 ± 22 | [117] |
| Poly(trimethylene carbonate dimethacrylate) hydrogel (PTMC-dame) | physical | 1.04 ± 0.04 | 0.46 ± 0.07 | 159 ± 43 | [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, G.; Niedźwiedź, M.J.; Kantor-Malujdy, N.; El Fray, M. Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications. Polymers 2022, 14, 1822. https://doi.org/10.3390/polym14091822
Demirci G, Niedźwiedź MJ, Kantor-Malujdy N, El Fray M. Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications. Polymers. 2022; 14(9):1822. https://doi.org/10.3390/polym14091822
Chicago/Turabian StyleDemirci, Gokhan, Malwina J. Niedźwiedź, Nina Kantor-Malujdy, and Miroslawa El Fray. 2022. "Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications" Polymers 14, no. 9: 1822. https://doi.org/10.3390/polym14091822
APA StyleDemirci, G., Niedźwiedź, M. J., Kantor-Malujdy, N., & El Fray, M. (2022). Elastomer–Hydrogel Systems: From Bio-Inspired Interfaces to Medical Applications. Polymers, 14(9), 1822. https://doi.org/10.3390/polym14091822