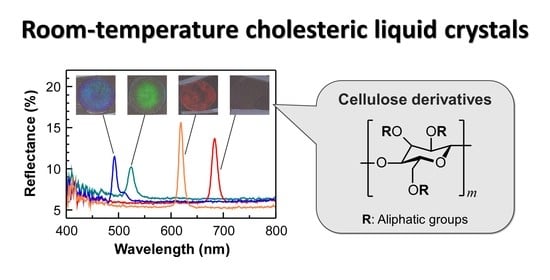
Room-Temperature Cholesteric Liquid Crystals of Cellulose Derivatives with Visible Reflection
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Synthesis and Characterization of HPC-X/Cn
3.2. Reflection Properties of HPC-X (X = -Pr, -Bu, and -Pe)
3.3. Side-Chain Length Effect of HPC-Pr/Cn, -Bu/Cn, and -Pe/Cn on Reflection Properties
3.4. Side-Chain Length Effect of HPC-X/C2 on Reflection Properties
3.5. Room-Temperature Thermotropic CLCs of HPC Derivatives with Visible Reflection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokhena, T.C.; John, M.J. Cellulose Nanomaterials: New Generation Materials for Solving Global Issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Nasseri, R.; Deutschman, C.P.; Han, L.; Pope, M.A.; Tam, K.C. Cellulose Nanocrystals in Smart and Stimuli-Responsive Materials: A Review. Mater. Today Adv. 2020, 5, 100055. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong Nanocomposite Reinforcement Effects in Polyurethane Elastomer with Low Volume Fraction of Cellulose Nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.J.; Xu, F.; Sun, R.C. Revealing Strong Nanocomposite Hydrogels Reinforced by Cellulose Nanocrystals: Insight into Morphologies and Interactions. ACS Appl. Mater. Interfaces 2013, 5, 12960–12967. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Wang, Y.; Liu, Y.; Liu, Y.; Chen, Y. New Nanocomposite Materials Reinforced with Cellulose Nanocrystals in Nitrile Rubber. Polym. Test. 2013, 32, 819–826. [Google Scholar] [CrossRef]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Werbowyj, R.S.; Gray, D.G. Liquid Crystalline Structure in Aqueous Hydroxypropyl Cellulose Solutions. Mol. Cryst. Liq. Cryst. 1976, 34, 97–103. [Google Scholar] [CrossRef]
- Bhadani, S.N.; Gray, D.G. Cellulose-Based Liquid Crystalline Polymers; Esters of (Hydroxypropyl) Cellulose. Mol. Cryst. Liq. Cryst. 1983, 99, 29–38. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Flexible and Iridescent Chiral Nematic Mesoporous Organosilica Films. J. Am. Chem. Soc. 2012, 134, 867–870. [Google Scholar] [CrossRef]
- Hayata, K.; Suzuki, T.; Fukawa, M.; Furumi, S. Thermotropic Cholesteric Liquid Crystals from Cellulose Derivatives with Ester and Carbamate Groups. J. Photopolym. Sci. Technol. 2019, 32, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Hayata, K.; Furumi, S. Side Chain Effect of Hydroxypropyl Cellulose Derivatives on Reflection Properties. Polymers 2019, 11, 1696. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Hayata, K.; Furumi, S. Cholesteric Liquid Crystals from Cellulose Derivatives with Alkyl Ether Groups. J. Photopolym. Sci. Technol. 2020, 33, 461–465. [Google Scholar] [CrossRef]
- Chan, C.L.C.; Lei, I.M.; Van De Kerkhof, G.T.; Parker, R.M.; Richards, K.D.; Evans, R.C.; Huang, Y.Y.S.; Vignolini, S. 3D Printing of Liquid Crystalline Hydroxypropyl Cellulose—Toward Tunable and Sustainable Volumetric Photonic Structures. Adv. Funct. Mater. 2022, 32, 2108566. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals in Living Matter. Soft Matter 2017, 13, 4176–4209. [Google Scholar] [CrossRef]
- Scarangella, A.; Soldan, V.; Mitov, M. Biomimetic Design of Iridescent Insect Cuticles with Tailored, Self-Organized Cholesteric Patterns. Nat. Commun. 2020, 11, 4108. [Google Scholar] [CrossRef]
- López, C. Materials Aspects of Photonic Crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- de Vries, H. Rotatory Power and Other Optical Properties of Certain Liquid Crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Aoki, R.; Fukawa, M.; Furumi, S. Preparation of the Color Films from Cellulose Derivatives in a Diacrylate Liquid. J. Photopolym. Sci. Technol. 2019, 32, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Kosho, H.; Hiramatsu, S.; Nishi, T.; Tanaka, Y.; Kawauchi, S.; Watanabe, J. Thermotropic Cholesteric Liquid Crystals in Ester Derivatives of Hydroxypropylcellulose. High Perform. Polym. 1999, 11, 41–48. [Google Scholar] [CrossRef]
- Asada, T.; Toda, K.; Onogi, S. Deformation and Structural Re-Formation of Lyotropic Cholesteric Liquid Crystal of Hydroxypropyl Cellulose + Water System. Mol. Cryst. Liq. Cryst. 1981, 68, 231–246. [Google Scholar] [CrossRef]
- Hussain, S.; Park, S.Y. Photonic Cholesteric Liquid-Crystal Elastomers with Reprogrammable Helical Pitch and Handedness. ACS Appl. Mater. Interfaces 2021, 13, 59275–59287. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.; Valenzuela, C.; Zhang, X.; Wang, L.; Feng, W. Mechanochromic, Shape-Programmable and Self-Healable Cholesteric Liquid Crystal Elastomers Enabled by Dynamic Covalent Boronic Ester Bonds. Angew. Chem. Int. Ed. 2022, 61, e202116219. [Google Scholar]
- Geng, Y.; Kizhakidathazhath, R.; Lagerwall, J.P.F. Robust Cholesteric Liquid Crystal Elastomer Fibres for Mechanochromic Textiles. Nat. Mater. 2022, 21, 1441–1447. [Google Scholar] [CrossRef]
- Moreira, M.F.; Carvalho, I.C.S.; Cao, W.; Bailey, C.; Taheri, B.; Palffy-Muhoray, P. Cholesteric Liquid-Crystal Laser as an Optic Fiber-Based Temperature Sensor. Appl. Phys. Lett. 2004, 85, 2691–2693. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, Y.; Luo, D. Optical Thermal Sensor Based on Cholesteric Film Refilled with Mixture of Toluene and Ethanol. Opt. Express 2017, 25, 26349–26355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Froyen, A.A.F.; Schenning, A.P.H.J.; Zhou, G.; Debije, M.G.; Haan, L.T. Temperature-Responsive Photonic Devices Based on Cholesteric Liquid Crystals. Adv. Photonics Res. 2021, 2, 2100016. [Google Scholar] [CrossRef]
- Manabe, T.; Sonoyama, K.; Takanishi, Y.; Ishikawa, K.; Takezoe, H. Toward Practical Application of Cholesteric Liquid Crystals to Tunable Lasers. J. Mater. Chem. 2008, 18, 3040–3043. [Google Scholar] [CrossRef]
- Furumi, S.; Tamaoki, N. Glass-Forming Cholesteric Liquid Crystal Oligomers for New Tunable Solid-State Laser. Adv. Mater. 2010, 22, 886–891. [Google Scholar] [CrossRef]
- Wenzlik, D.; Ohm, C.; Serra, C.; Zentel, R. Preparation of Cholesteric Particles from Cellulose Derivatives in a Microfluidic Setup. Soft Matter 2011, 7, 2340–2344. [Google Scholar] [CrossRef]
- Yamagishi, T.A.; Guittard, F.; Godinho, M.H.; Martins, A.F.; Cambon, A.; Sixou, P. Comparison of Thermal and Cholesteric Mesophase Properties among the Three Kind of Hydroxypropylcellulose (HPC) Derivatives. Polym. Bull. 1994, 32, 47–54. [Google Scholar] [CrossRef]
- Huang, B.; Ge, J.J.; Li, Y.; Hou, H. Aliphatic Acid Esters of (2-Hydroxypropyl) Cellulose —Effect of Side Chain Length on Properties of Cholesteric Liquid Crystals. Polymer 2007, 48, 264–269. [Google Scholar] [CrossRef]
- Ho, F.F.L.; Kohler, R.R.; Ward, G.A. Determination of Molar Substitution and Degree of Substitution of Hydroxypropyl Cellulose by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1972, 44, 178–181. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uenuma, S.; Furumi, S. Thermotropic Properties of Cholesteric Liquid Crystal from Hydroxypropyl Cellulose Mixed Esters. Kobunshi Ronbunshu 2015, 72, 737–745. [Google Scholar] [CrossRef]
- Hou, H.; Reuning, A.; Wendorff, J.H.; Greiner, A. Tuning of the Pitch Height of Thermotropic Cellulose Esters. Macromol. Chem. Phys. 2000, 201, 2050–2054. [Google Scholar] [CrossRef]
- Yamagishi, T.A.; Sixou, P. Preparation and Characteristics of Cholesteric Gel from Pentyl Ether of Hydroxypropyl Cellulose. Polymer 1995, 36, 2315–2317. [Google Scholar] [CrossRef]
- Tamaoki, N. Cholesteric Liquid Crystals for Color Information Technology. Adv. Mater. 2001, 13, 1135–1147. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Iwata, N.; Furumi, S. Viscoelastic Properties of Cholesteric Liquid Crystals from Hydroxypropyl Cellulose Derivatives. J. Photopolym. Sci. Technol. 2021, 34, 537–542. [Google Scholar] [CrossRef]
- Ohlendorf, P.; Greiner, A. Synthesis of Liquid Crystalline Thioether-Functionalized Hydroxypropyl Cellulose Esters. Polym. Chem. 2015, 6, 2734–2739. [Google Scholar] [CrossRef]






| Sample | Amounts of Reagents (eq.) | PrE | CnE | |
|---|---|---|---|---|
| C2H5COCl | CnH2n+1COCl | |||
| HPC-Pr | 5.40 | 0.00 | 3.00 | − |
| HPC-Pr/C3 | 5.10 | 0.30 | 2.72 | 0.33 |
| HPC-Pr/C4 | 5.10 | 0.30 | 2.71 | 0.33 |
| HPC-Pr/C5 | 5.10 | 0.30 | 2.71 | 0.30 |
| HPC-Pr/C6 | 5.10 | 0.30 | 2.70 | 0.34 |
| HPC-Pr/C7 | 5.10 | 0.30 | 2.67 | 0.35 |
| HPC-Pr/C9 | 5.10 | 0.30 | 2.68 | 0.34 |
| HPC-Pr/C11 | 5.10 | 0.30 | 2.69 | 0.34 |
| HPC-Pr/C13 | 5.10 | 0.30 | 2.68 | 0.35 |
| HPC-Pr/C15 | 5.10 | 0.30 | 2.69 | 0.33 |
| HPC-Pr/C17 | 5.10 | 0.30 | 2.73 | 0.31 |
| Sample | Amounts of Reagents (eq.) | BuE | CnE | |
|---|---|---|---|---|
| C3H7COCl | CnH2n+1COCl | |||
| HPC-Bu/C2 | 5.10 | 0.30 | 2.63 | 0.29 |
| HPC-Bu | 5.40 | 0.00 | 2.92 | − |
| HPC-Bu/C4 | 5.10 | 0.30 | 2.48 | 0.31 |
| HPC-Bu/C5 | 5.10 | 0.30 | 2.61 | 0.31 |
| HPC-Bu/C6 | 5.10 | 0.30 | 2.69 | 0.32 |
| HPC-Bu/C7 | 5.10 | 0.30 | 2.75 | 0.32 |
| HPC-Bu/C9 | 5.10 | 0.30 | 2.59 | 0.32 |
| HPC-BuC11 | 5.10 | 0.30 | 2.56 | 0.31 |
| HPC-Bu/C13 | 5.10 | 0.30 | 2.56 | 0.32 |
| HPC-Bu/C15 | 5.10 | 0.30 | 2.78 | 0.31 |
| HPC-Bu/C17 | 5.10 | 0.30 | 2.61 | 0.32 |
| Sample | Amounts of Reagents (eq.) | PeE | CnE | |
|---|---|---|---|---|
| C4H9COCl | CnH2n+1COCl | |||
| HPC-Pe/C2 | 5.10 | 0.30 | 2.71 | 0.32 |
| HPC-Pe/C3 | 5.10 | 0.30 | 2.45 | 0.32 |
| HPC-Pe | 5.40 | 0.00 | 2.85 | − |
| HPC-Pe/C5 | 5.10 | 0.30 | 2.44 | 0.31 |
| HPC-Pe/C6 | 5.10 | 0.30 | 2.43 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogiwara, Y.; Suzuki, T.; Iwata, N.; Furumi, S. Room-Temperature Cholesteric Liquid Crystals of Cellulose Derivatives with Visible Reflection. Polymers 2023, 15, 168. https://doi.org/10.3390/polym15010168
Ogiwara Y, Suzuki T, Iwata N, Furumi S. Room-Temperature Cholesteric Liquid Crystals of Cellulose Derivatives with Visible Reflection. Polymers. 2023; 15(1):168. https://doi.org/10.3390/polym15010168
Chicago/Turabian StyleOgiwara, Yuki, Tatsuya Suzuki, Naoto Iwata, and Seiichi Furumi. 2023. "Room-Temperature Cholesteric Liquid Crystals of Cellulose Derivatives with Visible Reflection" Polymers 15, no. 1: 168. https://doi.org/10.3390/polym15010168
APA StyleOgiwara, Y., Suzuki, T., Iwata, N., & Furumi, S. (2023). Room-Temperature Cholesteric Liquid Crystals of Cellulose Derivatives with Visible Reflection. Polymers, 15(1), 168. https://doi.org/10.3390/polym15010168