Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources
Abstract
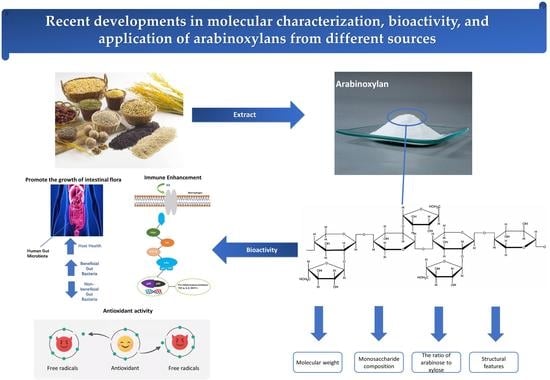
:1. Introduction
2. Molecular Characterization of AX from Different Sources
2.1. Molecular Weight
2.2. Monosaccharide Composition
2.3. Structural Feature
| AX Source | Extraction Method | Molecular Weight/kDa | Monosaccharide Composition (mol%) | Ara/Xyl | Structural Feature | Reference | |
|---|---|---|---|---|---|---|---|
| Barley | Hulless barley bran | 0.375 mol/L NaOH | 298.36 | Ara:Xyl:Gal:Glc: Man = 30.13:51.55:10.33:5.09:2.90 | 0.58 | 14.78% O-3 is monosubstituted, 10.76% O-2,3 is substituted | [35] |
| Barley hulls | 1 mol/L NaOH of 5% NaBH4 | 4300 | Ara:Xyl: Glc = 13.1:55.9:28.3 | 0.23 | / | [36] | |
| Peeled barley seeds | 1% NaBH4 in saturated Ba(OH)2 | 1360 | Ara:Xyl: Glc = 30.3:48.5:2.7 | 0.60 | Monosubstituted (O-2/3, 22.1%) and doubly substituted (O-2,3, 18.4%) | [37] | |
| Wheat | Wheat flour | 0.26 mol/L NaBH4 in saturated Ba(OH)2 | 2000 | Ara:Xyl:Gal:Glc: Man = 46.6:48.6:1.3:2.5:1.0 | 0.96 | β-The xylan backbone is present on the (1→4) bond and is substituted at O-3 or O-2 and O-3. | [38] |
| Wheat bran | 0.44 mol/LNaOH | / | Ara:Xyl:Gal:Glc:Man: Rha = 27.8:29.7:2.0:2.9:0.1:0.1 | 0.94 | / | [39] | |
| Rye | Rye grain | Hot water extraction | 156 | Ara:Xyl:Glc = 24.5:52.9:1.3: | 0.46 | / | [32] |
| Rye grain | NaOH extraction | 309 | Ara:Xyl:Gal:Glc: Man = 20.9:44.4:0.3:20.3:1.8 | 0.47 | / | ||
| Rye bran | 1% (w/v) NaBH4 in saturated Ba(OH)2 | 380 | Ara:Xyl: Glc: = 36.53:61.31:2.16 | 0.60 | The xylan skeleton contained 57.71% unsubstituted xylan residues and 6.22% disubstituted xylose. | [40] | |
| Sorghum | Sorghum seeds | 1% (w/v) NaBH4 in saturated Ca(OH)2 | 223.9 | Ara:Xyl:Gal:Glc:Man: Rha = 47.53:43.82:2.34:4.81:1.11:0.39 | 1.09 | The polysaccharide backbone is 1,4-β- D -xylan, which is replaced by α- l -arabinose residues mainly at the O-2 or O-3 sites | [41] |
| Sorghum bran | 126.6 | Ara:Xyl:Gal:Glc:Man: Rha = 49.37:45.45:0.13:2.74:1.78:0.52 | 1.09 | The polysaccharide backbone is 1,4-β- D-xylan, which is mainly substituted by α- l -arabinose residues at the O-2 or O-3 sites | |||
| Lacquer sorghum bran | Alkali extraction | 363 | Ara:Xyl:Gal:Glc:Rha:GalA: GlcA = 34.60:48.85:3.07:8.13:0.98:1.00;3.37 | 0.71 | / | [42] | |
| Corn | Corn Bran | Alkali extraction | 362 | Ara:Xyl:Gal:Glc:Rha:GalA: GlcA = 27.46:48.52:12.08:4.28:0.43:1.02:6.21 | 0.56 | / | [42] |
| Corn stover | 367 | Ara:Xyl:Gal:Glc:Rha:GalA: GlcA= 18.14:52.69:10.94:9.66:1.33:1.77:5.47 | 0.34 | / | |||
| Oats | Oat grain | 0.26 mol/L NaBH4 of saturated Ba(OH)2 | 6–2000 | Ara:Xyl:Gal:Glc: UA = 27:43:3:2:4 | 0.43 | / | [43] |
| Oat grain | 0.26 mol/L NaBH4 of 6 mol/L NaOH | 100 | Ara:Xyl:Gal:Glc: UA = 9:78:1:10:2 | 0.11 | / | ||
| Dicotyledonous plants | Pangola grass | Hydroborates | / | Ara:Xyl:Gal: Glc = 32.9:42.8:20.7:3.7 | 0.77 | Highly branched arabinoxylan, 60% xylose double substitution | [44] |
| Andrographis paniculate | 4% NaOH | 149 | Ara: Xyl = 20:80 | 0.25 | Constructed as the 1,4-alpha-D-xylose backbone | [45] | |
| Lauraeae | Water lifting | 175 | Ara:Xyl = 74:26 | 2.85 | Constructed as the 1,4-alpha-D-xylose backbone, substituted with furan arabinose at C2 | [46] |
3. Biological Activity of AX from Various Origin Sources
3.1. AX from Wheat
3.2. AX from Barley
3.3. AX from Sorghum
3.4. AX from Rice
3.5. AX from Corn
3.6. AX from Other Plants
| AX Source | Bioactivity | Experimental Subjects/Experimental Method | Biochemical Parameters | Results | Reference | |
|---|---|---|---|---|---|---|
| Wheat | Argentine soft wheat, Argentine durum wheat | Prebiotics | C57BL/6 male rat | Intestinal flora, SCFA | ↑Intestinal flora, ↑SCFA | [47] |
| Argentine soft wheat, Argentine durum wheat | Prebiotics | In vitro fermentation | pH, Gas production pressure, SCFA, Bifidobacteria, Lactobacillus abundance | ↓pH, ↑Gas production pressure, ↑SCFA, ↑Bifidobacteria, Lactobacillus abundance | [49] | |
| Wheat bran | Immunomodulation | Human monocytes | IL-6, TNF-α | ↑IL-6, ↑TNF-α | [50] | |
| Argentine soft wheat, Argentine durum wheat | Antitumor | HCT-116 colon cancer cells, macrophages, splenocytes | Cellular Viability | ↓Cellular Viability | [51] | |
| Barley | Barley flour | Hypoglycemic, prebiotic | C57BL/6J male mice | GLP-1, SCFA, Cecal chyme intestinal flora | ↑GLP-1, ↑SCFA, ↑Cecal chyme intestinal flora | [16] |
| Barley leaf | Immunomodulation | C3H/HeN rats | IgA; TGF-b1, GMCSF, IL-6 | ↑IgA; ↑TGF-b1, ↑GMCSF, ↑IL-6 | [52] | |
| Sorghum | Sorghum bran | Antioxidant activity | In vitro antioxidant assay | ORAC | High antioxidant capacity | [55] |
| Rice | Rice bran | Antitumor | Male Wistar rats | p53, Bax, Bcl-2, caspase-3, NF-κB/p65 | ↑p53, ↑Bax, ↓Bcl-2, ↑caspase-3, ↓NF-κB/p65 | [56] |
| Rice bran | Antitumor | Peripheral blood mononuclear cells; Mice | Cytotoxicity, TNF-α, IL- 6, IL-8, | ↑Cytotoxicity, ↑TNF-α, ↑IL- 6, ↑IL-8, | [8] | |
| Skimmed rice bran | Antioxidant activity | HepG2 cells (cellular antioxidant assay) | DPPH clearance rate, Fe2+ reducing ability, ROS | Stronger DPPH clearance and Fe2+ reduction | [57] | |
| Corn | Corn Bran | Prebiotics | In vitro fecal fermentation | Gas production, SCFA | ↑Gas production, ↑SCFA | [58] |
| Corn Bran | Prebiotics | Male Wistar rats | Appendix quality, SCFA, pH | ↑Appendix quality, ↑SCFA, ↓pH | [59] | |
| Corn fiber | Antioxidant activity | In vitro antioxidant assay | ORAC | Stronger resistance to oxidation | [60] | |
| Other Plants | Andrographis paniculata | Antioxidant activity | In vitro antioxidant assay | Fe2+ chelating ability, superoxide radical scavenging rate, hydroxyl radical scavenging rate | Stronger resistance to oxidation | [45] |
4. Applications
4.1. Flour Products
4.2. Wine
4.3. Films
4.4. Other Fields
5. AX Related Products
5.1. Food
5.2. Film Products
5.3. Other Products
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izydorczyk, M.S.; Biliaderis, C.G. Influence of structure on the physicochemical properties of wheat arabinoxylan. Carbohydr. Polym. 1992, 17, 237–247. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Romero, A.; Alonso, E. Extraction of arabinoxylans from wheat bran using hydrothermal processes assisted by heterogeneous catalysts. Carbohydr. Polym. 2016, 160, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; Smith, C.; Moda, H. Cereal-Derived Arabinoxylans as Biological Response Modifiers: Extraction, Molecular Features, and Immune-Stimulating Properties. Crit. Rev. Food Sci. Nutr. 2014, 55, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C. Synthesis and properties of feruloyl corn bran arabinoxylan esters. Int. J. Cosmet. Sci. 2015, 38, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors influencing their antioxidant capacity. Bioact. Carbohydr. Diet. Fibre 2017, 10, 20–26. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Sahasrabudhe, N.; Rösch, C.; Schols, H.A.; Faas, M.; de Vos, P. The impact of dietary fibers on dendritic cell responses IN VITRO is dependent on the differential effects of the fibers on intestinal epithelial cells. Mol. Nutr. Food Res. 2015, 59, 698–710. [Google Scholar] [CrossRef]
- Perez-Martinez, A.; Valentín, J.; Fernández, L.; Hernández-Jiménez, E.; López-Collazo, E.; Zerbes, P.; Schwörer, E.; Nuñéz, F.; Martín, I.; Sallis, H.; et al. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell–mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 2014, 17, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Savitha Prashanth, M.R.; Shruthi, R.R.; Muralikrishna, G. Immunomodulatory activity of purified arabinoxylans from finger millet (Eleusine coracana, v. Indaf 15) bran. J. Food Sci. Technol. 2015, 52, 6049–6054. [Google Scholar] [CrossRef]
- Damen, B.; Verspreet, J.; Pollet, A.; Broekaert, W.; Delcour, J.; Courtin, C. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011, 55, 1862–1874. [Google Scholar] [CrossRef]
- Grootaert, C.; Delcour, J.; Courtin, C.; Broekaert, W.; Verstraete, W.; Van de Wiele, T. Microbial metabolism and prebiotic potency of Arabinoxylan oligosaccharides in the human intestine. Trends Food Sci. Technol. 2007, 18, 64–71. [Google Scholar] [CrossRef]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylans, gut microbiota and immunity. Carbohydr. Polym. 2015, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.; Thakkar, R.; Arioglu-Tuncil, S.; Hamaker, B.; Lindemann, S. Fecal Microbiota Responses to Bran Particles Are Specific to Cereal Type and In Vitro Digestion Methods That Mimic Upper Gastrointestinal Tract Passage. J. Agric. Food Chem. 2018, 66, 12580–12593. [Google Scholar] [CrossRef] [PubMed]
- Rumpagaporn, P.; Reuhs, B.L.; Kaur, A.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr. Polym. 2015, 130, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Amrein, T.M.; Gränicher, P.; Arrigoni, E.; Amadò, R. In vitro digestibility and colonic fermentability of aleurone isolated from wheat bran. LWT—Food Sci. Technol. 2003, 36, 451–460. [Google Scholar] [CrossRef]
- Mio, K.; Ogawa, R.; Tadenuma, N.; Aoe, S. Arabinoxylan as well as β-glucan in barley promotes GLP-1 secretion by increasing short-chain fatty acids production. Biochem. Biophys. Rep. 2022, 32, 101343. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, H.; Kong, X.; Zhang, N.; Li, H.; Dong, X.; Li, Z. Oat β-Glucan Ameliorates Diabetes in High Fat Diet and Streptozotocin-induced Mice by Regulating Metabolites. J. Nutr. Biochem. 2022, 109251. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef]
- Rizzi, J.; Moro, T.R.; Winnischofer, S.M.B.; Colusse, G.A.; Tamiello, C.S.; Trombetta-Lima, M.; Noleto, G.R.; Dolga, A.M.; Duarte, M.E.R.; Noseda, M.D. Chemical structure and biological activity of the (1 → 3)-linked β-D-glucan isolated from marine diatom Conticribra weissflogii. Int. J. Biol. Macromol. 2023, 224, 584–593. [Google Scholar] [CrossRef]
- Cui, Y.; Han, X.; Huang, X.; Xie, W.; Zhang, X.; Zhang, Z.; Yu, Q.; Tao, L.; Li, T.; Li, S. Effects of different sources of β-glucan on pasting, gelation, and digestive properties of pea starch. Food Hydrocoll. 2023, 135, 108172. [Google Scholar] [CrossRef]
- Wang, P.; Tao, H.; Jin, Z.; Xu, X. Impact of water extractable arabinoxylan from rye bran on the frozen steamed bread dough quality. Food Chem. 2016, 200, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, R.; Qian, H.; Li, Y.; Zhang, H.; Qi, X.; Wang, L. Investigation the influences of water-extractable and water-unextractable arabinoxylan on the quality of whole wheat you-tiao and its mechanism. Food Chem. 2022, 386, 132809. [Google Scholar] [CrossRef]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of physicochemical properties, pre-processing, and extraction on the functionality of wheat bran arabinoxylans in breadmaking–A review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef]
- Pihlajaniemi, V.; Mattila, O.; Koitto, T.; Nikinmaa, M.; Heiniö, R.-L.; Sorsamäki, L.; Siika-aho, M.; Nordlund, E. Production of syrup rich in arabinoxylan oligomers and antioxidants from wheat bran by alkaline pretreatment and enzymatic hydrolysis, and applicability in baking. J. Cereal Sci. 2020, 95, 103043. [Google Scholar] [CrossRef]
- Sárossy, Z.; Tenkanen, M.; Pitkänen, L.; Bjerre, A.-B.; Plackett, D. Extraction and chemical characterization of rye arabinoxylan and the effect of β-glucan on the mechanical and barrier properties of cast arabinoxylan films. Food Hydrocoll. 2013, 30, 206–216. [Google Scholar] [CrossRef]
- He, H.J.; Qiao, J.; Liu, Y.; Guo, Q.; Ou, X.; Wang, X. Isolation, Structural, Functional, and Bioactive Properties of Cereal Arabinoxylan─A Critical Review. J. Agric. Food Chem. 2021, 69, 15437–15457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Schupfer, E.; Pak, S.C.; Wang, S.; Micalos, P.S.; Jeffries, T.; Ooi, S.L.; Golombick, T.; Harris, G.; El-Omar, E. The effects and benefits of arabinoxylans on human gut microbiota—A narrative review. Food Biosci. 2021, 43, 101267. [Google Scholar] [CrossRef]
- Comino, P. Effects of diverse food processing conditions on the structure and solubility of wheat, barley and rye endosperm dietary fibre. J. Food Eng. 2015, 169, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, M. Barley Arabinoxylans: Molecular, Physicochemical, and Functional Properties; American Associate of Cereal Chemists International: Saint Paul, MN, USA, 2014; pp. 97–122. [Google Scholar]
- Hromádková, Z.; Paulsen, B.S.; Polovka, M.; Košťálová, Z.; Ebringerová, A. Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydr. Polym. 2013, 93, 22–30. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R.; Praznik, W. Molecular properties of arabinoxylan fractions isolated from rye grain of different quality. J. Cereal Sci. 2014, 60, 368–373. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millan, E.; Rascon, A.; Márquez-Escalante, J.; Guerrero, V.; Salas-Muñoz, E. Feruloylated arabinoxylans and arabinoxylan gels: Structure, sources and applications. Phytochem. Rev. 2009, 9, 111–120. [Google Scholar] [CrossRef]
- Höije, A.; Sandström, C.; Roubroeks, J.P.; Andersson, R.; Gohil, S.; Gatenholm, P. Evidence of the presence of 2-O-β-d-xylopyranosyl-α-l-arabinofuranose side chains in barley husk arabinoxylan. Carbohydr. Res. 2006, 341, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xu, Z.; Wu, S.; Li, X.; Li, J.; Hu, H.; Wu, Y.; Ai, L. Molecular properties and structural characterization of an alkaline extractable arabinoxylan from hull-less barley bran. Carbohydr Polym 2019, 218, 250–260. [Google Scholar] [CrossRef]
- Hoije, A.; Grondahl, M.; Tommeraas, K.; Gatenholm, P. Isolation and characterization of physicochemical and material properties of arabinoxylans from barley husks. Carbohydr. Polym. 2005, 61, 266–275. [Google Scholar] [CrossRef]
- Li, L.Y.; Wang, Y.X.; Zhang, T.; Zhang, J.F.; Pan, M.; Huang, X.J.; Yin, J.Y.; Nie, S.P. Structural characteristics and rheological properties of alkali-extracted arabinoxylan from dehulled barley kernel. Carbohydr Polym. 2020, 249, 116813. [Google Scholar] [CrossRef] [PubMed]
- Revanappa, S.B.; Nandini, C.D.; Salimath, P.V. Structural variations of arabinoxylans extracted from different wheat (Triticum aestivum) cultivars in relation to chapati-quality. Food Hydrocoll. 2015, 43, 736–742. [Google Scholar] [CrossRef]
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, S.; Gu, X.; Zhang, J. Isolation and structural characterization of water unextractable arabinoxylans from Chinese black-grained wheat bran. Carbohydr. Polym. —CARBOHYD POLYM 2011, 85, 615–621. [Google Scholar] [CrossRef]
- Miafo, A.-P.T.; Muralikrishna, G.; Koubala, B.B.; Kansci, G. Purification and structural characterization of calcium hydroxide isolated arabinoxylans derived from bran, spent grain and sorghum grains. J. Cereal Sci. 2021, 100, 103266. [Google Scholar] [CrossRef]
- Kale, M.; Yadav, M.; Hicks, K.; Hanah, K. Concentration and shear rate dependence of solution viscosity for arabinoxylans from different sources. Food Hydrocoll. 2015, 47, 178–183. [Google Scholar] [CrossRef]
- Lingmin, T.; Harry, G.; Schols, H.A. Characterization of (Glucurono)arabinoxylans from Oats Using Enzymatic Fingerprinting. J. Agric. Food Chem. 2015, 63, 10822–10830. [Google Scholar]
- Ford, C.W. A feruloylated arabinoxylan liberated from cell walls of Digitaria decumbens (pangola grass) by treatment with borohydride. Carbohydr. Res. 1989, 190, 137–144. [Google Scholar] [CrossRef]
- Maity, G.N.; Maity, P.; Dasgupta, A.; Acharya, K.; Dalai, S.; Mondal, S. Structural and antioxidant studies of a new arabinoxylan from green stem Andrographis paniculata (Kalmegh). Carbohydr. Polym. 2019, 212, 297–303. [Google Scholar] [CrossRef]
- Das, D.; Maiti, S.; Maiti, T.K.; Islam, S.S. A new arabinoxylan from green leaves of Litsea glutinosa (Lauraeae): Structural and biological studies. Carbohydr. Polym. 2013, 92, 1243–1248. [Google Scholar] [CrossRef]
- Paesani, C.; Degano, A.L.; Salvucci, E.; Zalosnik, M.I.; Fabi, J.o.P.; Sciarini, L.S.; Perez, G.T. Soluble arabinoxylans extracted from soft and hard wheat show a differential prebiotic effect in vitro and in vivo. J. Cereal Sci. 2020, 93, 102956. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Paesani, C.; Sciarini, L.S.; Moiraghi, M.; Salvucci, E.; Prado, S.B.R.d.; Pérez, G.T.; Fabi, J.P.J.L.-F.S. Human colonic in vitro fermentation of water-soluble arabinoxylans from hard and soft wheat alters Bifidobacterium abundance and short-chain fatty acids concentration. LWT 2020, 134, 110253. [Google Scholar] [CrossRef]
- Moerings, B.G.J.; van Bergenhenegouwen, J.; Furber, M.; Abbring, S.; Schols, H.A.; Witkamp, R.F.; Govers, C.; Mes, J.J. Dectin-1b activation by arabinoxylans induces trained immunity in human monocyte-derived macrophages. Int J Biol Macromol 2022, 209, 942–950. [Google Scholar] [CrossRef]
- Paesani, C.; Degano, A.L.; Zalosnik, M.I.; Fabi, J.P.; Pérez, G.T. Enzymatic modification of arabinoxylans from soft and hard Argentinian wheat inhibits the viability of HCT-116 cells. Food Res. Int. (Ott. Ont.) 2021, 147, 110466. [Google Scholar] [CrossRef]
- Kim, H.; Yu, K.-W.; Hong, H.D.; Shin, K.S. Effect of arabinoxylan- and rhamnogalacturonan I-rich polysaccharides isolated from young barley leaf on intestinal immunostimulatory activity. J. Funct. Foods 2017, 35, 384–390. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Hong, H.-D.; Shin, K.-S. Rapid Isolation Method for Preparation of Immuno-Stimulating Rhamnogalacturonans in Citrus Peels. Korean J. Food Sci. Technol. 2015, 47, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J.; Gutierrez-Uribe, J.A. Phenolic compounds, antioxidant capacity and gelling properties of glucoarabinoxylans from three types of sorghum brans. J. Cereal Sci. 2015, 65, 277–284. [Google Scholar] [CrossRef]
- Badr El-Din, N.K.; Ali, D.A.; Othman, R.; French, S.W.; Ghoneum, M. Chemopreventive role of arabinoxylan rice bran, MGN-3/Biobran, on liver carcinogenesis in rats. Biomed Pharmacother. 2020, 126, 110064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Shao, J.-J.; Wang, Z.-L.; Lu, Z.-X. Study of allicin microcapsules in β-cyclodextrin and porous starch mixture. Food Res. Int. 2012, 49, 641–647. [Google Scholar] [CrossRef]
- Xu, H.; Reuhs, B.L.; Cantu-Jungles, T.M.; Tuncil, Y.E.; Kaur, A.; Terekhov, A.; Martens, E.C.; Hamaker, B.R. Corn arabinoxylan has a repeating structure of subunits of high branch complexity with slow gut microbiota fermentation. Carbohydr. Polym. 2022, 289, 119435. [Google Scholar] [CrossRef]
- Lopez, H.W.; Levrat, M.A.; Guy, C.; Messager, A.; Demigné, C.; Rémésy, C. Effects of soluble corn bran arabinoxylans on cecal digestion, lipid metabolism, and mineral balance (Ca, Mg) in rats. J. Nutr. Biochem. 1999, 10, 500–509. [Google Scholar] [CrossRef]
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; García-Lara, S.; Pérez-Carrillo, E. Hydroxycinnamic acids, sugar composition and antioxidant capacity of arabinoxylans extracted from different maize fiber sources. Food Hydrocoll. 2014, 35, 471–475. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Hussain, A.; MacGregor, A.W. Effect of Barley and Barley Components on Rheological Properties of Wheat Dough. J. Cereal Sci. 2001, 34, 251–260. [Google Scholar] [CrossRef]
- Frederix, S.A.; Van Hoeymissen, K.E.; Courtin, C.M.; Delcour, J.A. Water-extractable and water-unextractable arabinoxylans affect gluten agglomeration behavior during wheat flour gluten-starch separation. J. Agric. Food Chem. 2004, 52, 7950–7956. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; van Vliet, T.; Hamer, R.J. How gluten properties are affected by pentosans. J. Cereal Sci. 2004, 39, 395–402. [Google Scholar] [CrossRef]
- Agnieszka, N.; Szymanska-Chargot, M.; Miś, A.; Ptaszyńska, A.; Kowalski, R.; Wasko, P.; Gruszecki, W. Influence of dietary fibre on gluten proteins structure—A study on model flour with application of FT-Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 309–316. [Google Scholar] [CrossRef]
- Shelat, K.; Vilaplana, F.; Nicholson, T.; Wong, K.; Gidley, M. Diffusion and viscosity in arabinoxylan solutions: Implications for nutrition. Carbohydr. Polym. 2010, 82, 46–53. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schutter, D.P.; Courtin, C.M. Arabinoxylan from non-malted cereals can act as mouthfeel contributor in beer. Carbohydr. Polym. 2020, 239, 116257. [Google Scholar] [CrossRef] [PubMed]
- Stoklosa, R.; Latona, R.; Bonnaillie, L.; Yadav, M. Evaluation of Arabinoxylan Isolated from Sorghum Bran, Biomass, and Bagasse for Film Formation. Carbohydr. Polym. 2019, 213, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Simsek, S. Mechanical profiles and topographical properties of films made from alkaline extracted arabinoxylans from wheat bran, maize bran, or dried distillers grain. Food Hydrocoll. 2019, 86, 78–86. [Google Scholar] [CrossRef]
- Egüés, I.; Stepan, A.M.; Eceiza, A.; Toriz, G.; Gatenholm, P.; Labidi, J. Corncob arabinoxylan for new materials. Carbohydr. Polym. 2014, 102, 12–20. [Google Scholar] [CrossRef]
- Ali, U.; Kanwar, S.; Yadav, K.; Basu, S.; Mazumder, K. Effect of arabinoxylan and β-glucan stearic acid ester coatings on post-harvest quality of apple (Royal Delicious). Carbohydr. Polym. 2019, 209, 338–349. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, L.; Chen, F.; Yin, L.; Zhu, T.; Jie, Y. Wheat bran arabinoxylan and bovine serum albumin conjugates: Enzymatic synthesis, characterization, and applications in O/W emulsions. Food Res. Int. 2022, 158, 111452. [Google Scholar] [CrossRef]
- Pavlovich-Abril, A.; Rouzaud-Sández, O.; Carvajal-Millan, E.; Navarro, R.E.; Robles-Sánchez, R.M.; Barrón-Hoyos, J.M. Molecular characterization of water extractable arabinoxylans isolated from wheat fine bran and their effect on dough viscosity. LWT 2016, 74, 484–492. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Qiu, S.; Liu, C.; Zhang, P.; Yin, L.; Chen, F. Rheological properties and structural characteristics of arabinoxylan hydrogels prepared from three wheat bran sources. J. Cereal Sci. 2019, 88, 79–86. [Google Scholar] [CrossRef]

| Application | Main Component | Effect | Reference |
|---|---|---|---|
| Dough | WEAX, WUAX | WEAX improves the stability of the gluten protein network and makes dough form a uniform and dense gluten network. WUAX can protect gluten protein’s hydrogen and disulfide bond conformation | [62,63,64,65] |
| Youtiao | Wheat AX | Inhibits the formation of partial disulfide bonds and inhibits the thermal aggregation of gluten proteins | [22] |
| Beer | Rye flour, wheat malt | Improving beer viscosity | [66] |
| Film | Corn bran AX | Maximum tensile strength 29.3 MPa, tear resistance 0.3 N | [68] |
| Film | Corn cob AX | Young’s modulus increased to 1400–1600 MPa and strength increased to about 53 MPa | [69] |
| Fruit and vegetable composite coating film | AX, SABG | Extended storage period | [70] |
| O/W Emulsion | Wheat bran AX, bovine serum albumin | Enhanced stability, improved optimization performance | [71] |
| Patent Number | Name of Product | Main Component | Feature | |
|---|---|---|---|---|
| Food | CN111317093A | Flour for steamed buns | Wheat dextrin layer powder | Nutrient-rich, excellent dough processing performance, good product flavor and texture |
| CN112841568A | Fish balls with improved frost resistance and nutritional value | Surimi protein, starch, AX | High water-holding capacity, good frost resistance, high gel characteristics, low digestibility of starch | |
| CN110897023A | Wheat dextrin layer arabinoxylan hypoglycemic instant tea | Wheat AX, Barley AX, Green Barley AX, Hops, Orange Peel, Pu’er Tea Extract | Good taste, with a strong flavor, lowering blood sugar, safe and no side effects, suitable for a wide range of recipients | |
| Film Products | CN114058055A | Biodegradable frozen food packaging film with photothermal antibacterial function | Wheat bran AX | Easy to prepare, low cost, green and safe, photothermal thawing |
| CN114773689A | Ferulic acid-arabinoxylan copolymer antibacterial film | Ferulic acid-arabinoxylan copolymer | Good barrier performance to water vapor and oxygen, good antibacterial properties | |
| CN114015104A | Environmentally friendly food packaging film | AX | Anti-icing and anti-fouling accelerated freezing, biodegradable | |
| Other Products | CN113647609A | Compounding emulsifying thickener | Konjac gum, xanthan gum, sodium carboxymethyl cellulose, WUAX | Good water retention, adhesion, foam stability, and oxidation resistance |
| CN111955537A | Preservatives for shrimp dodgers | Rice bran protein-AX complex, lignan, arbutin | Inhibits the production of spoilage bacteria, and volatile salt nitrogen (TVB-N), and prevents the oxidation of unsaturated fatty acids, etc. | |
| CN112037962A | Direct printable transparent conductive paper | AX | Simple operation and high reproducibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.; Zhang, Y.; Tong, X.; Zhong, Y.; Kong, F.; Li, D.; Liu, X.; Qiao, Y. Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources. Polymers 2023, 15, 225. https://doi.org/10.3390/polym15010225
Pang J, Zhang Y, Tong X, Zhong Y, Kong F, Li D, Liu X, Qiao Y. Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources. Polymers. 2023; 15(1):225. https://doi.org/10.3390/polym15010225
Chicago/Turabian StylePang, Jinxin, Yi Zhang, Xiaoyang Tong, Yaoguang Zhong, Fanjun Kong, Dan Li, Xifan Liu, and Yongjin Qiao. 2023. "Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources" Polymers 15, no. 1: 225. https://doi.org/10.3390/polym15010225
APA StylePang, J., Zhang, Y., Tong, X., Zhong, Y., Kong, F., Li, D., Liu, X., & Qiao, Y. (2023). Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources. Polymers, 15(1), 225. https://doi.org/10.3390/polym15010225