Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
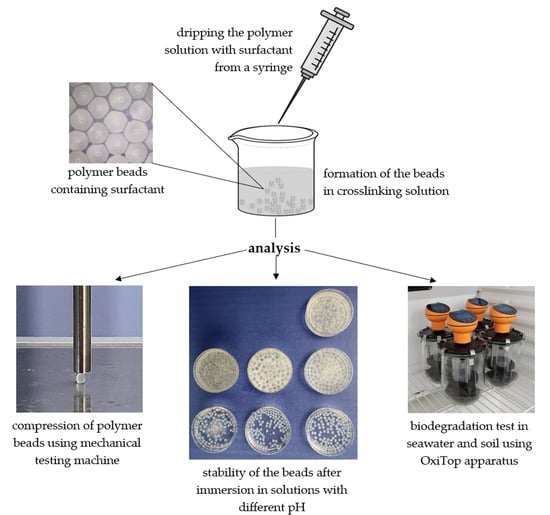
2.2. Polymer Bead Preparation
2.3. Studies of Macroparticles in Different Conditions
2.4. Mechanical Tests of Polymer Beads
2.5. Biodegradation Studies of Beads
3. Results and Discussion
3.1. Images and Size of Beads Immersed in Different pH Solutions
3.2. Mechanical Properties of Macroparticles
3.3. Biodegradation of the Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.R.; Zheng, R.; Tang, J.; Sun, H.J.; Wang, J. A mini-review on building insulation materials from perspective of plastic pollution: Current issues and natural fibres as a possible solution. J. Hazard. Mater. 2022, 438, 129449. [Google Scholar] [CrossRef] [PubMed]
- Singh Jadaun, J.; Bansal, S.; Sonthalia, A.; Rai, A.K.; Singh, S.P. Biodegradation of plastics for sustainable environment. Bioresour. Technol. 2022, 347, 126697. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lei, T.; Boré, A.; Lou, Z.; Abdouraman, B.; Ma, W. Evolution of global plastic waste trade flows from 2000 to 2020 and its predicted trade sinks in 2030. J. Clean. Prod. 2022, 376, 134373. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, X.; Ruan, J.; Wang, Y.; Zhang, J.; LeBlanc, G.A.; An, L. Ignored microplastic sources from plastic bottle recycling. Sci. Total Environ. 2022, 838, 156038. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, R.; Li, R. Impact of the COVID-19 pandemic on research on marine plastic pollution—A bibliometric-based assessment. Mar. Policy 2022, 146, 105285. [Google Scholar] [CrossRef]
- Maity, S.; Banerjee, S.; Biswas, C.; Guchhait, R.; Chatterjee, A.; Pramanick, K. Functional interplay between plastic polymers and microbes: A comprehensive review. Biodegradation 2021, 32, 487–510. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, X.; Li, M.; Li, Z.; Shi, J.; Huang, X.; Zou, X.; Yan, M.; Qian, W.; Gong, Y.; et al. Saccharomyces cerevisiae-incorporated and sucrose-rich sodium alginate film: An effective antioxidant packaging film for longan preservation. Int. J. Biol. Macromol. 2022, 223, 673–683. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Alharbi, K.S.; Almalki, W.H.; Imam, S.S.; Albratty, M.; Meraya, A.M.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Afzal, O.; et al. Sodium alginate based drug delivery in management of breast cancer. Carbohydr. Polym. 2022, 292, 119689. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, C.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Development of sodium alginate/glycerol/tannic acid coated cotton as antimicrobial system. Int. J. Biol. Macromol. 2022, 216, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Bae, S.B.; Nam, H.C.; Park, W.H. Electrospraying of environmentally sustainable alginate microbeads for cosmetic additives. Int. J. Biol. Macromol. 2019, 133, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Traffano-Schiffo, M.V.; Aguirre Calvo, T.R.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Alginate Beads Containing Lactase: Stability and Microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef]
- Kozlowska, J.; Prus, W.; Stachowiak, N. Microparticles based on natural and synthetic polymers for cosmetic applications. Int. J. Biol. Macromol. 2019, 129, 952–956. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Rodríguez, V.; Pérez-Esteban, P.; Martín del Valle, E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef]
- Das, M.; Giri, T.K. Hydrogels based on gellan gum in cell delivery and drug delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101586. [Google Scholar] [CrossRef]
- Wahba, M.I. Processed gellan gum beads as covalent immobilization carriers. Biocatal. Agric. Biotechnol. 2018, 14, 270–278. [Google Scholar] [CrossRef]
- Prezotti, F.G.; Cury, B.S.F.; Evangelista, R.C. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr. Polym. 2014, 113, 286–295. [Google Scholar] [CrossRef]
- Osmałek, T.; Milanowski, B.; Froelich, A.; Szybowicz, M.; Białowąs, W.; Kapela, M.; Gadziński, P.; Ancukiewicz, K. Design and characteristics of gellan gum beads for modified release of meloxicam. Drug Dev. Ind. Pharm. 2017, 43, 1314–1329. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Kim, G.Y.; Lee, M.Y.; Kim, Y.; Kang, S. Enhanced biodegradation of hydrocarbons by Pseudomonas aeruginosa-encapsulated alginate/gellan gum microbeads. J. Hazard. Mater. 2021, 406, 124752. [Google Scholar] [CrossRef]
- Jana, S.; Das, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded unsaturated esterified alginate/gellan gum microspheres: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2013, 57, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, N.R.; Goswami, A.K. Vildagliptin-loaded gellan gum mucoadhesive beads for sustained drug delivery: Design, optimisation and evaluation. Mater. Technol. 2020, 36, 647–659. [Google Scholar] [CrossRef]
- Carretta, L.; Cardinali, A.; Masin, R.; Zanin, G.; Cederlund, H. Decyl glucoside surfactant Triton CG-110 does not significantly affect the environmental fate of glyphosate in the soil at environmentally relevant concentrations. J. Hazard. Mater. 2020, 388, 122111. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Xiong, M.; Atwater, A.R.; DeKoven, J.G.; Pratt, M.D.; Maibach, H.I.; Taylor, J.S.; Belsito, D.V.; Silverberg, J.I.; Reeder, M.J.; et al. Patch testing with glucosides: The North American Contact Dermatitis Group experience, 2009–2018. J. Am. Acad. Dermatol. 2022, 87, 1033–1041. [Google Scholar] [CrossRef]
- Alfalah, M.; Loranger, C.; Sasseville, D. Alkyl Glucosides. Dermatitis 2017, 28, 3–4. [Google Scholar] [CrossRef]
- Severin, R.K.; Belsito, D.V. Patch Testing with Decyl and Lauryl Glucoside: How Well Does One Screen for Contact Allergic Reactions to the Other? Dermatitis 2017, 28, 342–345. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and Antibacterial Properties of Alginate Films Containing Tansy (Tanacetum vulgare L.) Essential Oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef]
- Wang, C.X.; Cowen, C.; Zhang, Z.; Thomas, C.R. High-speed compression of single alginate microspheres. Chem. Eng. Sci. 2005, 60, 6649–6657. [Google Scholar] [CrossRef]
- Olderøy, M.O.; Xie, M.; Andreassen, J.P.; Strand, B.L.; Zhang, Z.; Sikorski, P. Viscoelastic properties of mineralized alginate hydrogel beads. J. Mater. Sci. Mater. Med. 2012, 23, 1619–1627. [Google Scholar] [CrossRef]
- Kanesaka, S.; Watanabe, T.; Matsukawa, S. Binding effect of Cu2+ as a trigger on the sol-to-gel and the coil-to-helix transition processes of polysaccharide, gellan gum. Biomacromolecules 2004, 5, 863–868. [Google Scholar] [CrossRef]
- Singh, B.N.; Kim, K.H. Effects of divalent cations on drug encapsulation efficiency of deacylated gellan gum. J. Microencapsul. 2005, 22, 761–771. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Adrover, A.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Trilli, J.; Cesa, S.; Tho, I.; Casadei, M.A. Gellan gum/laponite beads for the modified release of drugs: Experimental and modeling study of gastrointestinal release. Pharmaceutics 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, J.A.P.; Perrechil, F.D.A.; Picone, C.S.F.; Sato, A.C.K.; Cunha, R.L. Da Preparation, characterization and in vitro digestibility of gellan and chitosan-gellan microgels. Carbohydr. Polym. 2015, 117, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fattah, E.A.E.; Grant, D.J.W.; Gabr, K.E.; Meshali, M.M. Physical characteristics and release behavior of salbutamol sulfate beads prepared with different ionic polysaccharides. Drug Dev. Ind. Pharm. 1998, 24, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, A.; Krasucka, P.; Charmas, B.; Stefaniak, W.; Goworek, J. Swelling effects in cross-linked polymers by thermogravimetry. J. Therm. Anal. Calorim. 2017, 130, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation behavior of polymers used as coating materials for drug delivery—A basic review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ruan, H.; Ma, L.L.; Wang, W.; Zhou, P.; He, G.Q. Study on swelling model and thermodynamic structure of native konjac glucomannan. J. Zhejiang Univ. Sci. B 2009, 10, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metze, F.K.; Sant, S.; Meng, Z.; Klok, H.A.; Kaur, K. Swelling-Activated, Soft Mechanochemistry in Polymer Materials. Langmuir 2022, 39, 3546–3557. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Lim, T.K.; Voo, W.P.; Pogaku, R.; Tey, B.T.; Zhang, Z. Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Sikorska, W.; Musiol, M.; Zawidlak-Wegrzynska, B.; Rydz, J. Compostable polymeric ecomaterials: Environment-friendly waste management alternative to landfills. In Handbook of Eco-Materials; Springer: Berlin/Heidelberg, Germany, 2019; Volume 4, ISBN 9783319682556. [Google Scholar]
- Gu, J.G.; Gu, J.D. Methods currently used in testing microbiological degradation and deterioration of a wide range of polymeric materials with various degree of degradability: A review. J. Polym. Environ. 2005, 13, 65–74. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Burkowska-But, A.; Tarach, I.; Walczak, M.; Jakubowska, E. Biodegradation of polylactide-based composites with an addition of a compatibilizing agent in different environments. Int. Biodeterior. Biodegrad. 2019, 147, 104840. [Google Scholar] [CrossRef]
- Richert, A.; Kalwasińska, A.; Brzezinska, M.S.; Dąbrowska, G.B. Biodegradability of novel polylactide and polycaprolactone materials with bacteriostatic properties due to embedded birch tar in different environments. Int. J. Mol. Sci. 2021, 22, 10228. [Google Scholar] [CrossRef]
- Ahsan, W.A.; Hussain, A.; Lin, C.; Nguyen, M.K. Biodegradation of Different Types of Bioplastics through Composting—A Recent Trend in Green Recycling. Catalysts 2023, 13, 294. [Google Scholar] [CrossRef]
- Wang, G.X.; Huang, D.; Ji, J.H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef]
- Park, S.H.; Kae, K.K.; Lee, D.S.; Hong, K.L. Morphological diversity of marine microorganisms on different isolation media. J. Microbiol. 2002, 40, 161–165. [Google Scholar]
- Meena, V.S.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Strength of Microbes in Nutrient Cycling: A Key to Soil Health. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1, pp. 1–356. [Google Scholar] [CrossRef]






| Solutions | Changes in Size of the Beads (%) after 24 h of Immersion in Different pH Solutions | ||
|---|---|---|---|
| ALG | GG | ALG + GG | |
| pH = 4 | −13.8 * ± 2.8 | −20.3 * ± 0.4 | −20.4 * ± 3.8 |
| pH = 7 | 29.2 ± 1.7 | 5.5 ± 2.4 | 6.1 ± 2.4 |
| pH = 8 | 39.0 ± 1.7 | 8.3 ± 2.0 | 11.0 ± 2.2 |
| pH = 10 | − | 11.5 ± 2.4 | 11.1 ± 2.7 |
| Solutions | Young’s Modulus (kPa) | ||
|---|---|---|---|
| ALG | GG | ALG + GG | |
| pH = 4 | 162.6 ± 8.4 a | 168.2 ± 9.9 a | 122.5 ± 6.9 b |
| pH = 5 | 149.9 ± 5.1 a | 152.4 ± 7.8 a | 113.7 ± 5.8 b |
| pH = 6 | 84.1 ± 4.2 c | 132.7 ± 8.7 a | 105.9 ± 8.6 b |
| pH = 7 | 55.9 ± 7.9 c | 122.6 ± 7.5 a | 96.7 ± 6.8 b |
| pH = 8 | 30.1 ± 6.9 b | 112.5 ± 10.3 a | 91.9 ± 3.4 a |
| pH = 9 | - | 105.4 ± 3.4 a | 77.9 ± 7.6 b |
| pH = 10 | - | 94.8 ± 6.5 a | 43.1 ± 4.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak, N.; Kowalonek, J.; Kozlowska, J.; Burkowska-But, A. Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant. Polymers 2023, 15, 2568. https://doi.org/10.3390/polym15112568
Stachowiak N, Kowalonek J, Kozlowska J, Burkowska-But A. Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant. Polymers. 2023; 15(11):2568. https://doi.org/10.3390/polym15112568
Chicago/Turabian StyleStachowiak, Natalia, Jolanta Kowalonek, Justyna Kozlowska, and Aleksandra Burkowska-But. 2023. "Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant" Polymers 15, no. 11: 2568. https://doi.org/10.3390/polym15112568
APA StyleStachowiak, N., Kowalonek, J., Kozlowska, J., & Burkowska-But, A. (2023). Stability Studies, Biodegradation Tests, and Mechanical Properties of Sodium Alginate and Gellan Gum Beads Containing Surfactant. Polymers, 15(11), 2568. https://doi.org/10.3390/polym15112568