Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy
Abstract
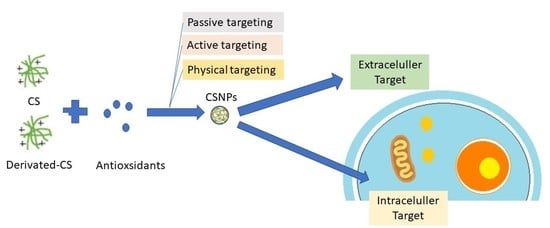
:1. Introduction
2. Antioxidants, Oxidative Stress, and Breast Cancer
2.1. Breast Cancer Overview
- Luminal A breast cancer is characterized by being hormone receptor positive (estrogen receptor and/or progesterone receptor positive) and HER2 negative. These cancers are typically low-grade, have a slow growth rate, and generally have a favorable prognosis.
- Luminal B breast cancer is hormone receptor positive (estrogen receptor and/or progesterone receptor positive) and can be either HER2 positive or HER2 negative. Luminal B cancers tend to grow slightly faster than the luminal A subtype.
- Triple-negative/basal-like breast cancer is hormone receptor negative (estrogen receptor and progesterone receptor negative) and HER2 negative. This type of cancer is more prevalent among younger women and African American women. Triple-negative breast cancer (TNBC) is particularly concerning, as over 50% of affected individuals may die within the first six months of developing metastatic disease.
- HER2-enriched breast cancer is hormone receptor negative (estrogen receptor and progesterone receptor negative) and HER2 positive. HER2-enriched cancers may have a poorer prognosis, but targeted therapies that specifically address the HER2 protein, such as trastuzumab, have proven to be effective treatments.
2.2. Oxidative Stress in Breast Cancer
2.3. Antioxidants in Breast Cancer
- The primary defense system of antioxidants comprises enzymes such as SOD, glutathione reductase (GR), catalase (CAT), and essential minerals such as zinc, selenium, and copper.
- The secondary defense system of antioxidants includes molecules such as glutathione (GSH), flavonoids, carotenoids, vitamin C, and vitamin E.
- The tertiary defense system of antioxidants involves a complex combination of chemicals responsible for repairing damaged DNA, proteins, oxidized lipids, and peroxides. Examples of these include DNA repair enzymes, methionine sulphoxide reductase, proteases, lipases, transferases, and other related substances [10].
2.4. Target Action of Antioxidants in Breast Cancer
- Intracellular targets: these targets involve mechanisms and pathways that are primarily located within the cancer cells themselves.
- (a)
- ROS;
- (b)
- Apoptosis;
- (c)
- Hormone receptor signaling;
- (d)
- Gene expression.
- Extracellular targets: these targets involve the microenvironment surrounding the cancer cells, including the extracellular matrix, stromal cells, and immune cells.
- (a)
- Inflammation;
- (b)
- Chemotherapy and radiotherapy side effects.
2.5. Strategies to Improve the Delivery of Antioxidants Using Chitosan
- Biocompatibility and Biodegradability: The vehicle should be non-toxic, non-immunogenic, and non-inflammatory, and should not elicit any adverse reactions or toxicity in the body.
- Enhanced Stability: The vehicle should protect the antioxidants from degradation and enhance their stability, particularly during storage and transportation, and be acid tolerant, and GI enzyme stable.
- Controlled Release: The vehicle should allow for a controlled release of antioxidants over a specific period, thereby enhancing their efficacy and duration of action.
- Optimal Bioavailability: The vehicle should improve the absorption and bioavailability of antioxidants, especially in the gastrointestinal (GI) tract, and extend the duration of contact between the drug and the mucosa to enhance drug absorption.
- Targeted Delivery: The vehicle should be designed to target specific sites in the body where antioxidants are required, thereby reducing systemic toxicity and enhancing therapeutic efficacy.
- Ease of Administration: The vehicle should be easy to administer, preferably orally, and should not require specialized equipment or expertise.
- Cost-Effective: The vehicle should be cost-effective and scalable, thereby enabling its widespread use and accessibility.
3. Delivery of Antioxidants Using Chitosan Nanoparticles
3.1. Preparation of Chitosan-Based Nanoparticles
3.1.1. Passive Targeting
- Crosslinked chitosan NPs
- 2.
- Chitosan-based polyelectrolyte complex NPs
- 3.
- Chitosan-coated nanoparticles
- 4.
- Chitosan nanocomposite
3.1.2. Physical Targeting
- Stimuli-sensitive chitosan-based nanoparticles
- 2.
- Magnetic chitosan-based nanoparticles
3.1.3. Active Targeting
3.2. Pharmacokinetic Properties Enhancement Delivery of Antioxidants Using Chitosan
3.2.1. Absorption
- Protection and stabilization: CSNPs such as enteric coatings can shield drugs from degradation within the harsh acidic conditions of the stomach. The NPs act as a protective barrier, preventing the drug from premature degradation and ensuring its integrity until it reaches the absorption site. This protection enables a higher concentration of the active drug to be available for absorption, thereby enhancing the overall absorption efficiency [110].
- Mucoadhesion: CSNPs exhibit mucoadhesive characteristics, enabling them to adhere to the mucosal surfaces of the GI tract. Through the interaction between the positively charged surface of CS and the negatively charged mucosal surfaces, these NPs promote extended contact between the NPs and the site of absorption. This extended contact enhances drug absorption by increasing the residence time and promoting closer interaction between the drug-loaded NPs and the underlying tissues.
- Permeability enhancement: CSNPs can enhance the permeability of drugs across the mucosal barriers of the GI tract. The presence of CS in the nanoparticle formulation can open up tight junctions between the epithelial cells, temporarily increasing the paracellular transport of drugs. This opening of tight junctions allows for improved drug diffusion and absorption through the intercellular spaces, leading to enhanced bioavailability [110].
- Increased surface area: CSNPs have a high surface-area-to-volume ratio due to their small particle size. This increased surface area provides more contact points between the drug-loaded NPs and the absorption site, facilitating efficient drug absorption. The larger surface area allows for greater interaction with the absorptive surfaces, increasing the chances of drug molecules being taken up into the systemic circulation [106].
- Modulation of drug release: CSNPs can be designed to achieve controlled and sustained drug release profiles. By encapsulating drugs within the NPs, their release can be modified and extended over time. This controlled release pattern ensures a gradual and consistent availability of the drug at the absorption site, optimizing the absorption efficiency and reducing the potential for dose dumping [33,51,100].
- Targeted release: The release of the payload from these nanocarriers is selectively activated by the presence of intestinal alkaline phosphatase (IAP), an enzyme located on the cell membrane. These virus-mimicking nanocarriers possess a surface with a high density of anionic and cationic charges, allowing them to penetrate the mucus gel layer and achieve targeted release of their cargo directly at the epithelial cells [110].
- Efflux inhibition: This refers to the process of blocking or reducing the activity of efflux transporters, which are proteins that pump substances out of cells. By inhibiting these transporters, the absorption of certain substances can be increased [111].
3.2.2. Distribution
3.2.3. Metabolism
3.2.4. Excretion
4. Pharmacological Enhancement of Antioxidant and Anticancer Activity in Chitosan-Based Nanoparticles
4.1. Antioxidant Activity
4.2. Anticancer Activity
4.3. Antioxidant and Anticancer Activity Enhancement
5. Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 7–9. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Misganaw, M.; Zeleke, H.; Mulugeta, H.; Assefa, B. Mortality rate and predictors among patients with breast cancer at a referral hospital in northwest Ethiopia: A retrospective follow-up study. PLoS ONE 2023, 18, e0279656. [Google Scholar] [CrossRef]
- Barrios, C.H. Global challenges in breast cancer detection and treatment. Breast 2022, 62, S3–S6. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, R.M.; Bhattacharjee, D.; Somanna, P.; Jain, V. Role of Nrf2 Signaling Cascade in Breast Cancer: Strategies and Treatment. Front. Pharmacol. 2022, 13, 720076. [Google Scholar] [CrossRef]
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M.; et al. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Zou, L. Significant Role of Antioxidants in the Treatment of Breast Cancer. Oxid. Antioxid. Med. Sci. 2022, 11, 2022. [Google Scholar]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Akanji, M.A.; Fatinukun, H.D.; Rotimi, D.E.; Afolabi, B.L.; Adeyemi, O.S. The Two Sides of Dietary Antioxidants in Cancer Therapy. In Antioxidants; Waisundara, V., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 9; ISBN 978-1-83968-865-2. [Google Scholar]
- Wieland, L.S.; Moffet, I.; Shade, S.; Emadi, A.; Knott, C.; Gorman, E.F.; D’Adamo, C. Risks and benefits of antioxidant dietary supplement use during cancer treatment: Protocol for a scoping review. BMJ Open 2021, 11, e047200. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Wahabi, K.; Perwez, A.; Rizvi, M.A. Antioxidant in Cancer BT—Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Chakraborti, S., Ed.; Springer: Singapore, 2021; pp. 1–16. ISBN 978-981-16-1247-3. [Google Scholar]
- Krejbich, P.; Birringer, M. The Self-Administered Use of Complementary and Alternative Medicine (CAM) Supplements and Antioxidants in Cancer Therapy and the Critical Role of Nrf-2—A Systematic Review. Antioxidants 2022, 11, 2149. [Google Scholar] [CrossRef]
- Jung, A.Y.; Cai, X.; Thoene, K.; Obi, N.; Jaskulski, S.; Behrens, S.; Flesch-Janys, D.; Chang-Claude, J. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am. J. Clin. Nutr. 2019, 109, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Reitz, L.K.; Schroeder, J.; Longo, G.Z.; Boaventura, B.C.B.; Di Pietro, P.F. Dietary Antioxidant Capacity Promotes a Protective Effect against Exacerbated Oxidative Stress in Women Undergoing Adjuvant Treatment for Breast Cancer in a Prospective Study. Nutrients 2021, 13, 4324. [Google Scholar] [CrossRef]
- McGrowder, D.; Miller, F.; Nwokocha, C.; Wilson-Clarke, C.; Anderson, M.; Anderson-Jackson, L.; Williams, L. Micronutrient Antioxidants in the Chemoprevention of Breast Cancer and Effect on Breast Cancer Outcomes; Waisundara, V., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2; ISBN 978-1-83968-865-2. [Google Scholar]
- de Oliveira, V.A.; Oliveira, I.K.F.; Pereira, I.C.; Mendes, L.K.F.; da Silva, F.C.C.; Torres–Leal, F.L.; Sousa, J.M.d.C.e.; Paiva, A.d.A. Consumption and supplementation of vitamin E in breast cancer risk, treatment, and outcomes: A systematic review with meta-analysis. Clin. Nutr. ESPEN 2023, 54, 215–226. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered Tissues and Strategies to Overcome Challenges in Drug Development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Spruill, M.L.; Maletic-Savatic, M.; Martin, H.; Li, F.; Liu, X. Spatial analysis of drug absorption, distribution, metabolism, and toxicology using mass spectrometry imaging. Biochem. Pharmacol. 2022, 201, 115080. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, S.-I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Furniturewalla, A.; Barve, K. Approaches to overcome bioavailability inconsistencies of epigallocatechin gallate, a powerful anti-oxidant in green tea. Food Chem. Adv. 2022, 1, 100037. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2022, 2013, 353–374. [Google Scholar] [CrossRef] [Green Version]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in Medicine: A Focus on Vascular Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef] [Green Version]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. α-Mangostin Nanoparticles Cytotoxicity and Cell Death Modalities in Breast Cancer Cell Lines. Molecules 2021, 26, 5119. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs doped chitosan-based composite hydrogels as highly efficient catalysts for reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125889. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef]
- Ahghari, M.A.; Kamalzare, M.; Maleki, A. Design, synthesis, and characterization of novel eco-friendly chitosan-AgIO3 bionanocomposite and study its antibacterial activity. Sci. Rep. 2022, 12, 10491. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Cytotoxicity Enhancement in MCF-7 Breast Cancer Cells with Depolymerized Chitosan Delivery of α-Mangostin. Polymers 2022, 14, 3139. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; El Kadib, A. Chitosan as a sustainable precursor for nitrogen-containing carbon nanomaterials: Synthesis and uses. Mater. Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Zhu, Y.; Marin, L.M.; Xiao, Y.; Gillies, E.R.; Siqueira, W.L. pH-Sensitive Chitosan Nanoparticles for Salivary Protein Delivery. Nanomaterials 2021, 11, 1028. [Google Scholar] [CrossRef]
- Guadarrama-Escobar, O.R.; Serrano-Castañeda, P.; Anguiano-Almazán, E.; Vázquez-Durán, A.; Peña-Juárez, M.C.; Vera-Graziano, R.; Morales-Florido, M.I.; Rodriguez-Perez, B.; Rodriguez-Cruz, I.M.; Miranda-Calderón, J.E.; et al. Chitosan Nanoparticles as Oral Drug Carriers. Int. J. Mol. Sci. 2023, 24, 4289. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2021, 8, e08674. [Google Scholar] [CrossRef]
- Zhou, J.; Li, N.; Liu, P.; Liu, Z.; Gao, L.; Jiao, T. Preparation of Fluorescently Labeled Chitosan-Quercetin Drug-Loaded Nanoparticles with Excellent Antibacterial Properties. J. Funct. Biomater. 2022, 13, 141. [Google Scholar] [CrossRef]
- Zaffarin, A.S.M.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Seyam, S.; Alallam, B.; Doolaanea, A.A.; Alfatama, M. Current Advances in Chitosan Nanoparticles Based Oral Drug Delivery for Colorectal Cancer Treatment. Int. J. Nanomed. 2022, 17, 3933–3966. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J. Rizwanullah Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Francies, F.Z.; Hull, R.; Khanyile, R.; Dlamini, Z. Breast cancer in low-middle income countries: Abnormality in splicing and lack of targeted treatment options. Am. J. Cancer Res. 2020, 10, 1568–1591. [Google Scholar]
- Rakha, A.E.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2022, 82, 5–16. [Google Scholar] [CrossRef]
- Kunstič, T.T.; Debeljak, N.; Tacer, K.F. Heterogeneity in hormone-dependent breast cancer and therapy: Steroid hormones, HER2, melanoma antigens, and cannabinoid receptors. Adv. Cancer Biol.-Metastasis 2023, 7, 100086. [Google Scholar] [CrossRef]
- Zhao, N.; Rosen, J.M. Breast cancer heterogeneity through the lens of single-cell analysis and spatial pathologies. Semin. Cancer Biol. 2022, 82, 3–10. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Domenicotti, C.; Marengo, B. Paradox Role of Oxidative Stress in Cancer: State of the Art. Antioxidants 2022, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Han, R.; Liu, C.; Qin, X.; Li, P.; Gu, R. Signaling pathways of oxidative stress response: The potential therapeutic targets in gastric cancer. Front. Immunol. 2023, 14, 1139589. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Liu, Y.; Hao, C.; Li, L.; Zhang, H.; Zha, W.; Ma, L.; Chen, L. The Role of Oxidative Stress in the Development and Therapeutic Intervention of Hepatocellular Carcinoma. Curr. Cancer Drug Targets 2023, 23, 37073651. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-L.; Babuharisankar, A.P.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Mdkhana, B.; Goel, S.; Saleh, M.A.; Siddiqui, R.; Khan, N.A.; Elmoselhi, A.B. Role of oxidative stress in angiogenesis and the therapeutic potential of antioxidants in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4677–4692. [Google Scholar] [CrossRef]
- Dewage, E.; Sandun, N.; Nam, K.; Huang, X.; Ahn, D.U. Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1–18. [Google Scholar]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Narod, S.A.; Iqbal, J.; Miller, A.B. Why have breast cancer mortality rates declined? J. Cancer Policy 2015, 5, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Ladas, E.J.; Jacobson, J.S.; Kennedy, D.D.; Teel, K.; Fleischauer, A.; Kelly, K.M. Antioxidants and Cancer Therapy: A Systematic Review. J. Clin. Oncol. 2004, 22, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef] [Green Version]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Milella, R.A.; De Rosso, M.; Gasparro, M.; Gigante, I.; Debiase, G.; Forleo, L.R.; Marsico, A.D.; Perniola, R.; Tutino, V.; Notarnicola, M.; et al. Correlation between antioxidant and anticancer activity and phenolic profile of new Apulian table grape genotypes (V. vinifera L.). Front. Plant Sci. 2023, 13, 1064023. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal Prospects of Antioxidants from Algal Sources in Cancer Therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2022, 80, 241–262. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [Green Version]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef]
- Sobantu, M.P.; Okeleye, B.I.; Okudoh, V.I.; Meyer, M.; Aboua, Y.G. In Vitro Antioxidant Mechanism of Action of Hibiscus sabdariffa in the Induction of Apoptosis against Breast Cancer. J. Herbs Spices Med. Plants 2022, 29, 213–228. [Google Scholar] [CrossRef]
- Rahaman, M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef] [PubMed]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of Oxidative Stress in the Development and Progression of Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef]
- Bekhet, O.H.; Eid, M.E. The interplay between reactive oxygen species and antioxidants in cancer progression and therapy: A narrative review. Transl. Cancer Res. 2021, 10, 4196–4206. [Google Scholar] [CrossRef]
- Coughlin, S.S. Oxidative Stress, Antioxidants, Physical Activity, and the Prevention of Breast Cancer Initiation and Progression. J. Environ. Health Sci. 2018, 4, 55–57. [Google Scholar] [PubMed]
- Alqarni, A.A.; Alamoudi, A.A.; Allam, R.M.; Ajabnoor, G.M.; Harakeh, S.M.; Al-Abd, A.M. The influence of antioxidant dietary-derived polyphenolic combination on breast cancer: Molecular study. Biomed. Pharmacother. 2022, 149, 112835. [Google Scholar] [CrossRef]
- Andraos, C.; Gulumian, M. Intracellular and extracellular targets as mechanisms of cancer therapy by nanomaterials in relation to their physicochemical properties. WIREs Nanomed. Nanobiotechnol. 2020, 13, e1680. [Google Scholar] [CrossRef]
- Apostolova, N.; Victor, V.M.; Singh-Mallah, G.; Nair, S.; Sandberg, M.; Mallard, C.; Hagberg, H.; Rovira-Llopis, S.; Bañuls, C.; Muntané, J.; et al. Molecular Strategies for Targeting Antioxidants to Mitochondria: Therapeutic Implications. Antioxid. Redox Signal. 2015, 22, 686–729. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.Q.; Gong, Y.C.; Li, Z.L.; Li, Y.P.; Xiong, X.Y. Folate-conjugated pluronic/polylactic acid polymersomes for oral delivery of paclitaxel. Int. J. Biol. Macromol. 2019, 139, 377–386. [Google Scholar] [CrossRef]
- Seyam, S.; Nordin, N.A.; Alfatama, M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals 2020, 13, 307. [Google Scholar] [CrossRef]
- Kadian, R. Nanoparticles: A Promising Drug Delivery Approach. Asian J. Pharm. Clin. Res. 2018, 11, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Vieira, A.C.F.; Teixeira, J.; Gomes, M.J.; Barrocas, P.; Vasconcelos, T.; Sarmento, B. Cell-Based Intestinal In Vitro Models for Drug Absorption Screening BT-Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Hock, F.J., Gralinski, M.R., Pugsley, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–22. ISBN 978-3-030-73317-9. [Google Scholar]
- Sethi, A.; Ahmad, M.; Huma, T.; Khalid, I.; Ahmad, I. Evaluation of Low Molecular Weight Cross Linked Chitosan Nanoparticles, to Enhance the Bioavailability of 5-Flourouracil. Dose-Response 2021, 19, 15593258211025353. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Khurana, R.K.; Kesharwani, P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of Herbal Extracts in Treatment of Neurodegenerative Disorders. Front. Bioeng. Biotechnol. 2020, 8, 238. [Google Scholar] [CrossRef]
- Iacob, A.T.; Lupascu, F.G.; Apotrosoaei, M.; Vasincu, I.M.; Tauser, R.G.; Lupascu, D.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Recent Biomedical Approaches for Chitosan Based Materials as Drug Delivery Nanocarriers. Pharmaceutics 2021, 13, 587. [Google Scholar] [CrossRef]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E.B.T.-C.D.D. (Eds.) Chitosan-based polyelectrolyte complexes as pharmaceutical excipients. In Woodhead Publishing Series in Biomedicine; Woodhead Publishing: Sawston, UK, 2015; pp. 127–161. ISBN 978-1-907568-45-9. [Google Scholar]
- Lin, Y.; Wang, X.; Liu, Q.; Fang, Y. Preparation and Application of Chitosan-based Polyelectrolyte Complex Materials: An Overview. Pap. Biomater. 2022, 7, 1–19. [Google Scholar]
- Abuelella, K.E.; Abd-allah, H.; Soliman, S.M.; Abdel-mottaleb, M.M.A.; Pharmacy, I.; Pharmacy, I.; Shams, A. Bulletin of Pharmaceutical Sciences Nanoparticles: Preparation and Characterization. Bull. Pharm. Sci. 2022, 45, 53–62. [Google Scholar]
- Fong, S.S.; Foo, Y.Y.; Saw, W.S.; Leo, B.F.; Teo, Y.Y.; Chung, I.; Goh, B.T.; Misran, M.; Imae, T.; Chang, C.-C.; et al. Chitosan-Coated-PLGA Nanoparticles Enhance the Antitumor and Antimigration Activity of Stattic—A STAT3 Dimerization Blocker. Int. J. Nanomed. 2022, 17, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.; Ashrafudoulla; Parvez, A.K.; Balusamy, S.R.; Rahman, M.; Kim, J.H.; Akter, S. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.O.; Cunha, R.S.; Hotza, D.; Machado, R.A.F. Chitosan as a matrix of nanocomposites: A review on nanostructures, processes, properties, and applications. Carbohydr. Polym. 2021, 272, 118472. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Gozali, D.; Shamsuddin, S.; Muchtaridi, M. Chitosan-Based Nano-Smart Drug Delivery System in Breast Cancer Therapy. Pharmaceutics 2023, 15, 879. [Google Scholar] [CrossRef]
- Dung, D.T.K.; Hai, T.H.; Phuc, L.H.; Long, B.D.; Vinh, L.K.; Truc, P.N. Preparation and characterization of magnetic nanoparticles with chitosan coating. J. Phys. Conf. Ser. 2009, 187, 12036. [Google Scholar] [CrossRef]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Iswanti, F.C.; Nurulita, I.; Djauzi, S.; Sadikin, M.; Witarto, A.B.; Yamazaki, T. Preparation, characterization, and evaluation of chitosan-based nanoparticles as CpG ODN carriers. Biotechnol. Biotechnol. Equip. 2019, 33, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Wang, D.; Liu, D.; Su, J.; Jin, Y.; Wang, D.; Han, B.; Jiang, Z.; Liu, B. Applications of Chitosan and its Derivatives in Skin and Soft Tissue Diseases. Front. Bioeng. Biotechnol. 2022, 10, 894667. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Panigrahi, D.; Sahu, P.K.; Swain, S.; Verma, R.K. Quality by design prospects of pharmaceuticals application of double emulsion method for PLGA loaded nanoparticles. SN Appl. Sci. 2021, 3, 638. [Google Scholar] [CrossRef]
- Lifschitz, A.; Lanusse, C.; Alvarez, L. Host pharmacokinetics and drug accumulation of anthelmintics within target helminth parasites of ruminants. N. Z. Veter-J. 2017, 65, 176–184. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Saleh, A.; Akkuş-Dağdeviren, Z.B.; Friedl, J.D.; Knoll, P.; Bernkop-Schnürch, A. Chitosan—Polyphosphate nanoparticles for a targeted drug release at the absorption membrane. Heliyon 2022, 8, e10577. [Google Scholar] [CrossRef] [PubMed]
- Pathomthongtaweechai, N.; Muanprasat, C. Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics 2021, 13, 887. [Google Scholar] [CrossRef]
- Le, T.N.; Her, J.; Sim, T.; Jung, C.E.; Kang, J.K.; Oh, K.T. Preparation of Gastro-retentive Tablets Employing Controlled Superporous Networks for Improved Drug Bioavailability. AAPS PharmSciTech 2020, 21, 320. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, W. Drug metabolism in drug discovery and development. Acta Pharm. Sin. B 2018, 8, 721–732. [Google Scholar] [CrossRef]
- Shabbir, A.; Haider, K.; Rehman, K.; Akash, M.S.H.; Chen, S. Chapter 1—Biochemical Activation and Functions of Drug-Metabolizing Enzymes; Hamid Akash, M.S., Kanwal Rehman, Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–27. ISBN 978-0-323-95120-3. [Google Scholar]
- Barreto, E.F.; Larson, T.R.; Koubek, E.J.B.T.-R.M. Drug Excretion. In Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-801238-3. [Google Scholar]
- Yao, H.-T.; Lii, C.-K.; Chou, R.-H.; Lin, J.-H.; Yang, H.-T.; Chiang, M.-T. Effect of Chitosan on Hepatic Drug-Metabolizing Enzymes and Oxidative Stress in Rats Fed Low- and High-Fat Diets. J. Agric. Food Chem. 2010, 58, 5187–5193. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N. Gastrointestinal Delivery of APIs from Chitosan Nanoparticles. In Chitin and Chitosan; Berrada, M., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 2; ISBN 978-1-78984-425-2. [Google Scholar]
- Xiong, W.; Xiong, S.H.; Chen, Q.L.; Linghu, K.G.; Zhao, G.D.; Chu, J.M.; Wong, G.T.; Li, J.; Hu, Y.J.; Wang, Y.T.; et al. Brij-functionalized chitosan nanocarrier system enhances the intestinal permeability of P-glycoprotein substrate-like drugs. Carbohydr. Polym. 2021, 266, 118112. [Google Scholar] [CrossRef]
- Dong, W.; Han, B.; Feng, Y.; Song, F.; Chang, J.; Jiang, H.; Tang, Y.; Liu, W. Pharmacokinetics and Biodegradation Mechanisms of a Versatile Carboxymethyl Derivative of Chitosan in Rats: In Vivo and In Vitro Evaluation. Biomacromolecules 2010, 11, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Z.; Han, B.; Niu, S.; Dong, W.; Liu, W. Pharmacokinetics and biodegradation of chitosan in rats. J. Ocean Univ. China 2015, 14, 897–904. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Alallam, B.; Choukaife, H.; Seyam, S.; Lim, V.; Alfatama, M. Advanced Drug Delivery Systems for Renal Disorders. Gels 2023, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef]
- Martins, A.F.; Bueno, P.V.; Almeida, E.A.; Rodrigues, F.H.; Rubira, A.F.; Muniz, E.C. Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int. J. Biol. Macromol. 2013, 57, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Karewicz, A.; Bielska, D.; Loboda, A.; Gzyl-Malcher, B.; Bednar, J.; Jozkowicz, A.; Dulak, J.; Nowakowska, M. Curcumin-containing liposomes stabilized by thin layers of chitosan derivatives. Colloids Surf. B Biointerfaces 2013, 109, 307–316. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- van Pomeren, M.; Peijnenburg, W.; Vlieg, R.; van Noort, S.; Vijver, M. The biodistribution and immuno-responses of differently shaped non-modified gold particles in zebrafish embryos. Nanotoxicology 2019, 13, 558–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alphandéry, E. Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, M.; Ji, M.; Zhang, L.; Qin, Z.; Zhang, Y.; Gao, L.; Jiao, T. Magnetic graphene oxide-containing chitosan-sodium alginate hydrogel beads for highly efficient and sustainable removal of cationic dyes. Int. J. Biol. Macromol. 2021, 193, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2018, 126, 731–742. [Google Scholar] [CrossRef]
- Quester, K.; Rodríguez-González, S.; González-Dávalos, L.; Lozano-Flores, C.; González-Gallardo, A.; Zapiain-Merino, S.J.; Shimada, A.; Mora, O.; Vazquez-Duhalt, R. Chitosan Nanoparticles Containing Lipoic Acid with Antioxidant Properties as a Potential Nutritional Supplement. Animals 2022, 12, 417. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Yi, J.; Fan, Y.; Wu, X.; Zhang, Y. α-Lactalbumin and chitosan core–shell nanoparticles: Resveratrol loading, protection, and antioxidant activity. Food Funct. 2020, 11, 1525–1536. [Google Scholar] [CrossRef]
- Soleymanfallah, S.; Khoshkhoo, Z.; Hosseini, S.E.; Azizi, M.H. Preparation, physical properties, and evaluation of antioxidant capacity of aqueous grape extract loaded in chitosan-TPP nanoparticles. Food Sci. Nutr. 2022, 10, 3272–3281. [Google Scholar] [CrossRef]
- Kim, E.S.; Baek, Y.; Yoo, H.-J.; Lee, J.-S.; Lee, H.G. Chitosan-Tripolyphosphate Nanoparticles Prepared by Ionic Gelation Improve the Antioxidant Activities of Astaxanthin in the In Vitro and In Vivo Model. Antioxidants 2022, 11, 479. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Parize, A.L. In vitro cytotoxic and antioxidant evaluation of quercetin loaded in ionic cross-linked chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 74, 103561. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, S.-Y.; Lee, C.-M. Glycol Chitosan-Astaxanthin Nanoparticles: Water Dispersion, Antioxidant Activity, and Improved Cell Migration. Macromol. Res. 2022, 30, 712–718. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Epple, M.; Maskos, M. Stability of Nanoparticle Dispersions and Particle Agglomeration BT—Biological Responses to Nanoscale Particles: Molecular and Cellular Aspects and Methodological Approaches; Gehr, P., Zellner, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 85–100. ISBN 978-3-030-12461-8. [Google Scholar]
- Liu, Z.; Fu, R.; Yuying, Y. Chapter 2—Preparation and Evaluation of Stable Nanofluids for Heat Transfer Application; Ali, H.M.B.T.-A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–57. ISBN 978-0-323-88656-7. [Google Scholar]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.S.A.; Kwon, D.; Park, J.; Choi, S.Y.; Yoon, T.H.; Park, J. Effects of surface-modifying ligands on the colloidal stability of ZnO nanoparticle dispersions in in vitro cytotoxicity test media. Int. J. Nanomed. 2014, 9, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Gommes, C.J. Ostwald ripening of confined nanoparticles: Chemomechanical coupling in nanopores. Nanoscale 2019, 11, 7386–7393. [Google Scholar] [CrossRef]
- Awad, A.; Madla, C.M.; Gavins, F.K.H.; Allahham, N.; Trenfield, S.J.; Basit, A.W. Chapter 20—Liquid Dosage Forms, 23rd ed.; Adejare, A.B.T.-R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 359–379. ISBN 978-0-12-820007-0. [Google Scholar]
- Bucci, G.; Gadelrab, K.; Carter, W.C. Mesoscale Model for Ostwald Ripening of Catalyst Nanoparticles. J. Electrochem. Soc. 2021, 168, 054515. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, H.; Gao, J.; Zheng, A. Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv. 2020, 28, 19–36. [Google Scholar] [CrossRef]
- Liebig, F.; Thünemann, A.F.; Koetz, J. Ostwald Ripening Growth Mechanism of Gold Nanotriangles in Vesicular Template Phases. Langmuir 2016, 32, 10928–10935. [Google Scholar] [CrossRef]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research Strategies for Safety Evaluation of Nanomaterials, Part V: Role of Dissolution in Biological Fate and Effects of Nanoscale Particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Capek, I.B.T.-S. (Ed.) Chapter 4 Modification and Passivation of Colloidal Particles. In Nanocomposite Structures and Dispersions; Elsevier: Amsterdam, The Netherlands, 2006; Volume 23, pp. 225–292. ISBN 1383-7303. [Google Scholar]
- Zhang, M.; Shao, S.; Yue, H.; Wang, X.; Zhang, W.; Chen, F.; Zheng, L.; Xing, J.; Qin, Y. High Stability Au NPs: From Design to Application in Nanomedicine. Int. J. Nanomed. 2021, 16, 6067–6094. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y. Perspectives on important considerations in designing nanoparticles for oral delivery applications in food. J. Agric. Food Res. 2020, 2, 100031. [Google Scholar] [CrossRef]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef] [PubMed]



| Natural Antioxidant | Chitosan | Preparation | Characterization of NPs | Pharmacokinetic Enhancement | Pharmacologic Enhancement | Ref. |
|---|---|---|---|---|---|---|
| α-Mangostin | CS and thiolated CS (TCS) are crosslinked using genipin (GP), and the surface is then modified using Eudragit L100 | Crosslinked CSNPs | d CSNPs = 437–922 nm, d TCS-based NPs = 365–767 nm, both possessing positive charges on the surface. | TCS-based NPs with GP and L100 exhibit strong mucoadhesion to colon mucosa and provide an increase in α-mangostin loading for controlled-release drug delivery to the colon while limiting its release in the upper GI tract. | The active compound, α-mangostin, was released from NPs and showed effective anti-tumor activity against HT-29 colorectal cancer cells. The combination of pH-dependent and mucoadhesive properties in the TCS NPs allowed for specific delivery of α-mangostin to the colon site, resulting in anti-tumor activity. | [124] |
| Curcumin (CUR) | N-trimethyl CS/alginate BEADS complexes | Polyelectrolyte complexes (PECs) | The dry samples had an average diameter of around 0.10–0.20 mm. During drying, the surfaces of the beads folded, which created blockages in the material’s pores. | A controlled manner by using beads loaded with CUR as a drug carrier. The results were promising, with 100% of the CUR being released in the simulated intestinal fluid within 24 h. | The biological activity of CUR is enhanced when it is close to the physiological pH. | [125] |
| Curcumin | N-dodecyl CS-HPTMA chloride-coated liposomal | CS-coated NPs | ZP (31.6–32.3 mV) measurements confirmed the effective coating of liposomes with all these CS derivatives. Particle size 73.15 nm. | These NPs can easily enter the cell membrane and release curcumin in a controlled manner. | Liposomal curcumin showed higher uptake in tumor cells than normal cells, resulting in increased cytotoxicity towards B16F10 cancer cells without significant negative effects on normal cells. | [126] |
| Quercetin | TPP-chitosomes are a hybrid system consisting of liposomes coated with crosslinked CS. | CS-coated NPs | The nanocarriers were small, spherical particles (~180 nm) with high entrapment efficiency (~91%). | The protective polyelectrolyte shell layer shielded the vesicles and drug from stomach acidity. The system resisted acidity and released in alkaline pH such as the intestines. Quercetin release depended on pH (preferably alkaline) and was controlled by drug diffusion through the hybrid system. | [127] |
| Natural Antioxidant | Chitosan | Preparation | Characterization of NPs | Pharmacologic Effect Enhancement | Ref. |
|---|---|---|---|---|---|
| Peppermint oil (PO) and green tea oil (GTO) | CS-PO-NPs/CS-GTO NPs | Ionic gelation method mediated by TPP | The NPs had a spherical shape with an average size range of 20–60 nm (TEM). They exhibited a loading capacity of 22.2% for PO and 23.1% for GTO. The release of drugs in different buffer systems followed a Fickian behavior in vitro. | The NPs significantly improved the antioxidant activity, enhancing it approximately 2-fold for PO and 2.4-fold for GTO. | [134] |
| Alfa-lipoic acid (ALA) | ALA-CS-GFP-NPs | Ionic gelation in the presence of ALA | d = 44 nm and ZP = +32 mV. They effectively entered 3T3-L1 fibroblasts and crossed the intestinal barrier in vitro. ALA was released slowly from the NPs, indicating their stability in the stomach and subsequent absorption in the intestines. | Encapsulating ALA in CS-ALA-NPs did not alter its antioxidant activity. The CS-ALA-NPs retained their antioxidant activity and remained stable in simulated stomach conditions for up to 3 h. | [135] |
| Epigallocatechin-3-gallate (EGCG) | EGCG CNPs | Ionic gelation method mediated by TPP | The particle diameters of EGCG CNPs ranged from 41.31 to 388.36 nm. | Even a small concentration of 1.0 µg/mL of EGCG CNPs improved the antioxidant capacity and quality of Kacang buck semen after thawing. | [136] |
| Aqueous grape extract (AGE) | CS-AGE-NPs | Ionic gelation method mediated by TPP | The size of the NPs was 177.5 ± 2.12 nm, and they had a positive charge of 32.95 ± 0.49 mV. The CSNPs demonstrated good encapsulation efficiency and loading capacity. | The grape extract, when in its free form, exhibited antioxidant activity ranging from 15.6% to 51.01%. However, when the extract was encapsulated, its antioxidant activity increased further, ranging from 21.2% to 62.8%. | [137] |
| Resveratrol (RES)-loaded protein–polysaccharide NPs | RES–ALA–CSNPs | Oppositely charged α-lactalbumin (ALA) and chitosan (CS) interact through electrostatic forces | d RES–ALA–CHI NPs were 211.0 nm and Z = 13.23 mV. | The interaction between α-lactalbumin (ALA) and CS is based on simple electrostatic interactions between their opposite charges. | [136] |
| Natural Antioxidant | Chitosan | Preparation | Characterization of NPs | Antioxidant Enhancement | Anticancer Enhancement | Ref. |
|---|---|---|---|---|---|---|
| Naringenin (NAR) | CSNPs/NAR | Ionic gelation method by tripolyphosphate (TPP). | The native CSNPs had a size of 53.2 nm, which increased to 407.47 nm when loaded with NAR. The encapsulation efficiency of CSNPs/NAR was approximately 70% and 80% (HPLC method). Around 15% of the NAR was released from CSNPs/NAR, suggesting that the CSNPs effectively retained a high amount of NAR in the simulated gastric fluid (SGF), enhancing the drug’s bioavailability. | Using CSNPs/NAR at 0.3 mg/mL and 0.5 mg/mL led to a significant decrease in nitrite levels, reducing nitrate rates by 0.16 M and 0.12 M, respectively. NAR and BHT also showed significant reductions, with rates of 0.17 M and 0.19 M, respectively. | CS-encapsulated NAR was better than using free NAR alone. This highlights an effective system for delivering NAR with antioxidant and anticancer properties. | [41] |
| Astaxanthin (AST) | CS-AST-NPs | Ionic gelation method by tripolyphosphate (TPP). | d = 505.2 nm in size, Z = +20.4 mV, and showed uniformity in their size distribution. They effectively encapsulated around 63.9% of the drug. These NPs released the drug slowly, allowing it to stay in the bloodstream for a longer time. | The lipid peroxidation and DPPH assay results demonstrate that the ACT-NPs effectively preserved the antioxidant activity of ASX. | The ACT-NPs exhibited enhanced cytoprotective effects on the BHK-21 cell line, providing increased protection to the cells. | [138] |
| Quercetin (QUE) | CS-QUE-NPs | Ionic gelation method by tripolyphosphate (TPP). | PDI = 0.208, a hydrodynamic d = 103.2 nm, and a positive ZP of +30.4 mV. Quercetin encapsulation efficiency was 83.8%, and its release followed a gradual and faster pattern in pH 7.4 NaH2PO4 solutions through a non-Fickian mechanism. | The NPs exhibited a stronger antioxidant effect compared to free quercetin. | Quercetin encapsulated in NPs exhibited significant cytotoxicity against MCF-7 (human breast tumor) and A549 (human lung tumor) cells over a 72 h duration. | [139] |
| Astaxanthin (AXT) | Glycol CS (GC)-decorated AXT NPs (GC-AXT-NPs) | GC and AXT self-organize in water through ionic interactions. | The bioavailability of AXT could be enhanced by formulating AXT NPs that self-organize with GC. | The AXT NPs demonstrated higher inhibition effects on the production of nitric oxide (NO) and the secretion of prostaglandin E2 (PGE2) compared to AXT alone. | The GC-AXT NPs promoted cell migration and proliferation in L292 cells during scratch assays. Additionally, the viability of L929 fibroblast cells remained similar to that of normal cells, indicating no significant changes caused by the NPs. | [140] |
| Ag-NPs | CS-Ag-NPs composite | The NPs were synthesized using a chemical reduction process, with CS serving as both a reducing agent and a stabilizing agent. | d = 9–65 nm. | The NPs showed high antioxidant activity at different concentrations: 92%, 90%, and 75% at 4000, 2000, and 1000 µg/mL, respectively. The IC50 value, representing the concentration needed for 50% inhibition, was 261 µg/mL for Chi/Ag-NPs. | The NPs showed lower toxicity towards normal human skin cell line (BJ-1) cells compared to doxorubicin, which demonstrated higher toxicity. | [141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herdiana, Y.; Husni, P.; Nurhasanah, S.; Shamsuddin, S.; Wathoni, N. Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy. Polymers 2023, 15, 2953. https://doi.org/10.3390/polym15132953
Herdiana Y, Husni P, Nurhasanah S, Shamsuddin S, Wathoni N. Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy. Polymers. 2023; 15(13):2953. https://doi.org/10.3390/polym15132953
Chicago/Turabian StyleHerdiana, Yedi, Patihul Husni, Siti Nurhasanah, Shaharum Shamsuddin, and Nasrul Wathoni. 2023. "Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy" Polymers 15, no. 13: 2953. https://doi.org/10.3390/polym15132953
APA StyleHerdiana, Y., Husni, P., Nurhasanah, S., Shamsuddin, S., & Wathoni, N. (2023). Chitosan-Based Nano Systems for Natural Antioxidants in Breast Cancer Therapy. Polymers, 15(13), 2953. https://doi.org/10.3390/polym15132953