Magnetically Controlled Hyaluronic Acid–Maghemite Nanocomposites with Embedded Doxorubicin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
3. Results and Discussion
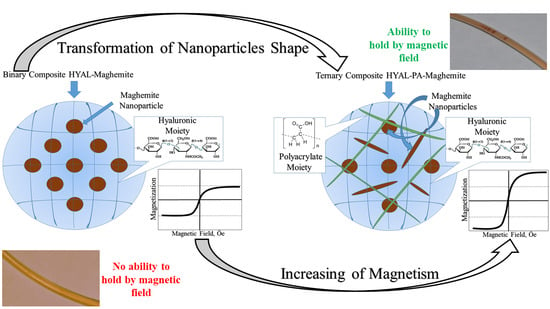
3.1. Synthesis of the Magnetic Binary Composites
3.2. Synthesis of HYAL-PA-Maghemite Ternary Composites
3.3. Magnetic Properties of the Polymer–Maghemite Composites
3.4. Antitumor Activity of Doxorubicin-Loaded Polymer–Maghemite Composites
3.5. Enzyme-Induced Degradation of the Polymer–Maghemite Composites
3.6. Enzyme-Induced Release of Doxorubicin from the Polymer–Maghemite Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef]
- Howes, P.; Green, M.; Bowers, A.; Parker, D.; Varma, G.; Kallumadil, M.; Botnar, R. Magnetic Conjugated Polymer Nanoparticles as Bimodal Imaging Agents. J. Am. Chem. Soc. 2010, 132, 9833–9842. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Shinkai, M.; Yanase, M.; Suzuki, M.; Hiroyuki, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Intracellular hyperthermia for cancer using magnetite cationic liposomes. J. Magn. Magn. Mater. 1999, 194, 176–184. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef]
- Phung, D.C.; Nguyen, H.T.; Phuong Tran, T.T.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar] [CrossRef]
- Shi, F.; Luo, D.; Zhou, X.; Sun, Q.; Shen, P.; Wang, S. Combined effects of hyperthermia and chemotherapy on the regulate autophagy of oral squamous cell carcinoma cells under a hypoxic microenvironment. Cell Death Discov. 2021, 7, 227. [Google Scholar] [CrossRef]
- Colombo, R.; Salonia, A.; Da Pozzo, L.; Naspro, R.; Freschi, M.; Paroni, R.; Rigatti, P. Combination of intravesical chemotherapy and hyperthermia for the treatment of superficial bladder cancer: Preliminary clinical experience. Crit. Rev. Oncol. Hematol. 2003, 47, 127–139. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, J.; Yu, J.; Zheng, Z.; Liu, F.; Yang, A. Recent advances of high performance magnetic iron oxide nanoparticles: Controlled synthesis, properties tuning and cancer theranostics. Nano Sel. 2020, 2, 216–250. [Google Scholar] [CrossRef]
- Chen, C.-L.; Zhang, H.; Ye, Q.; Hsieh, W.-Y.; Hitchens, T.K.; Shen, H.-H.; Ho, C. A New Nano-sized Iron Oxide Particle with High Sensitivity for Cellular Magnetic Resonance Imaging. Mol. Imaging Biol. 2010, 13, 825–839. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Martin, D.A.; Alvarez, V.A.; Gonzalez, J.S. Polyacrylic acid-coated iron oxide magnetic nanoparticles: The polymer molecular weight influence. Colloids Surf. A Physicochem. Eng. Asp. 2018, 543, 28–37. [Google Scholar] [CrossRef]
- Spiridonov, V.V.; Panova, I.G.; Makarova, L.A.; Zezin, S.B.; Novakova, A.A.; Baluyan, T.G.; Yaroslavov, A.A. Magneto-sensitive hybrid nanocomposites of water-soluble sodium alginate cross-linked with calcium ions and maghemite. Express Polym. Lett. 2018, 12, 452–461. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Safronov, A.P.; Zubarev, A.Y.; Shklyar, T.F.; Makeyev, O.G.; Makarova, E.B.; Kurlyandskaya, G.V. Polyacrylamide ferrogels with embedded maghemite nanoparticles for biomedical engineering. Results Phys. 2017, 7, 3624–3633. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 100163. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Rehbock, C.; Huhn, D.; Carrillo-Carrion, C.; de Aberasturi, D.J.; Merk, V.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef]
- Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Calero, M.; Gutiérrez, L.; Salas, G.; Luengo, Y.; Lázaro, A.; Acedo, P.; Villanueva, A. Efficient and safe internalization of magnetic iron oxide nanoparticles: Two fundamental requirements for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 733–743. [Google Scholar] [CrossRef]
- Liu, C.-G.; Han, Y.-H.; Zhang, J.-T.; Kumar Kankala, R.; Wang, S.-B.; Chen, A.-Z. Rerouting Engineered Metal-dependent Shapes of Mesoporous Silica Nanocontainers to Biodegradable Janus-type (Sphero-ellipsoid) Nanoreactors for Chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 1890–1905. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, S.; Mickoleit, F.; Jörke, C.; Clement, J.H.; Markert, S.; Jérôme, V.; Schenk, A.S. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications. Acta Biomater. 2020, 120, 293–303. [Google Scholar] [CrossRef]
- Takahashi, M.; Mohan, P.; Mukai, K.; Takeda, Y.; Matsumoto, T.; Matsumura, K.; Maenosono, S. Magnetic Separation of Autophagosomes from Mammalian Cells Using Magnetic–Plasmonic Hybrid Nanobeads. ACS Omega 2017, 2, 4929–4937. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Israel, L.L.; Ostrovsky, S.; Taylor, A.; Poptani, H.; Lellouche, J.-P.; Chari, D. Development of Multifunctional Magnetic Nanoparticles for Genetic Engineering and Tracking of Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 841–849. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Markiewicz, K.; Wilczewska, A.; Car, H. Magnetic nanoparticles as new diagnostic tools in medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef]
- Scarberry, K.E.; Dickerson, E.B.; McDonald, J.F.; Zhang, Z.J. Magnetic Nanoparticle−Peptide Conjugates for in Vitro and in Vivo Targeting and Extraction of Cancer Cells. J. Am. Chem. Soc. 2008, 130, 10258–10262. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Lee, S.-E.; Kim, D.-H.; Pyo, Y.-C.; Park, J.-S. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J. Pharm. Investig. 2019, 50, 261–270. [Google Scholar] [CrossRef]
- Spiridonov, V.V.; Panova, I.G.; Makarova, L.A.; Afanasov, M.I.; Zezin, S.B.; Sybachin, A.V.; Yaroslavov, A.A. The one-step synthesis of polymer-based magnetic γ-Fe2O3/carboxymethyl cellulose nanocomposites. Carbohydr. Polym. 2017, 177, 269–274. [Google Scholar] [CrossRef]
- Topchieva, I.N.; Spiridonov, V.V.; Kataeva, N.A.; Gubin, S.P.; Filippov, S.K.; Lezov, A.V. Magnetic nanocomposites based on cyclodextrin-containing molecular tubes and iron nanoparticles. Colloid Polym. Sci. 2006, 284, 795–801. [Google Scholar] [CrossRef]
- Spiridonov, V.; Panova, I.; Kusaia, V.; Makarova, L.; Romodina, M.; Fedyanin, A.; Pozdnyakova, N.; Shibaeva, A.; Yaroslavov, A. Doxorubicin Loaded Magnetosensitive Water-Soluble Nanogel Based on NIPAM and Iron (3+) Containing Nanoparticles. Macromol. Symp. 2020, 389, 1900072. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Wiener, J.; Farooq, A.; Saskova, J.; Noman, M.T. Development of Maghemite Glass Fibre Nanocomposite for Adsorptive Removal of Methylene Blue. Fibers Polym. 2018, 19, 1735–1746. [Google Scholar] [CrossRef]
- Herynek, V.; Babič, M.; Kaman, O.; Charvátová, H.; Veselá, M.; Buchholz, O.; Vosmanská, M.; Kubániová, D.; Kohout, J.; Hofmann, U.G.; et al. Maghemite nanoparticles coated by methacrylamide-based polymer for magnetic particle imaging. J. Nanoparticle Res. 2021, 23, 52. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Martínez, A.I.; Anaya, A.O.; Valladares, L.D.L.S.; Félix, L.L.; Dominguez, A.B. Structural and Magnetic Properties of Monophasic Maghemite (γ-Fe2O3) Nanocrystalline Powder. Adv. Nanopart. 2014, 3, 114–121. [Google Scholar] [CrossRef]
- Horner, O.; Neveu, S.; de Montredon, S.; Siaugue, J.-M.; Cabuil, V. Hydrothermal synthesis of large maghemite nanoparticles: Influence of the pH on the particle size. J. Nanopart. Res. 2009, 11, 1247. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Peng, T.; Zhou, J.; Ren, F.; Jiang, C. Synthesis and Magnetic Properties of Maghemite (γ-Fe2O3) Short-Nanotubes. Nanoscale Res. Lett. 2010, 5, 1474. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798. [Google Scholar] [CrossRef]
- Zachariah, M.R.; Aquino, M.I.; Shull, R.D.; Steel, E.B. Formation of superparamagnetic nanocomposites from vapor phase condensation in a flame. Nanostructured Mater. 1995, 5, 383–392. [Google Scholar] [CrossRef]
- Winsett, J.; Moilanen, A.; Paudel, K.; Kamali, S.; Ding, K.; Cribb, W.; Neupane, S. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef]
- Tuček, J.; Zboril, R.; Petridis, D. Maghemite Nanoparticles by View of Mössbauer Spectroscopy. J. Nanosci. Nanotechnol. 2006, 6, 926–947. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, G.M.; De Grave, E.; Bowen, L.H.; De Bakker, P.M.A.; Vandenberghe, R.E. Variable-temperature Mössbauer spectroscopy of nanosized maghemite and Al-substituted maghemites. Clays Clay Miner. 1995, 43, 562–568. [Google Scholar] [CrossRef]
- Haneda, K.; Morrish, A.H. On the hyperfine field of γ-Fe2O3 small particles. Phys. Letters. 1977, 64, 259–262. [Google Scholar] [CrossRef]
- Tronc, E.; Prene, P.; Jolivet, J.P.; d’Orazio, F.; Lucari, F.; Fiorani, D. Magnetic behaviour of γ-Fe2O3 nanoparticles by Mössbauer spectroscopy and magnetic measurements. Hyperfine Interact. 1995, 95, 129–148. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Bustamante, A.; Flores, J.; Mejía Santillan, M.; Osorio, A.M.; Martínez, A.I.; De Los Santos Valladares, L.; Barnes, C.H.W. Mössbauer study of intermediate superparamagnetic relaxation of maghemite (γ-Fe2O3) nanoparticles. Hyperfine Interact. 2013, 224, 89–97. [Google Scholar] [CrossRef]
- Coaquira, J.A.H.; Rodriguez, A.F.R.; Santos, J.G.; Silveira, L.B.; Oliveira, A.C. Magnetic characterization of maghemite nanoparticles dispersed in surface-treated polymeric template. Hyperfine Interact. 2007, 176, 113. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.-K.; Kim, J.-H.; Kweon, D.-K.; Lee, J.-W. Degradation of hyaluronic acid powder by electron beam irradiation, gamma ray irradiation, microwave irradiation and thermal treatment: A comparative study. Carbohydr. Polym. 2010, 79, 1080–1085. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; Silva, M.d.L.C.d.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2016, 182, 276–293. [Google Scholar] [CrossRef]
- El-Safory, N.S.; Lee, C.-K. Cytotoxic and antioxidant effects of unsaturated hyaluronic acid oligomers. Carbohydr. Polym. 2010, 82, 1116–1123. [Google Scholar] [CrossRef]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.E.; Zwanikken, J.W.; Olvera de la Cruz, M. Effect of Ion–Ion Correlations on Polyelectrolyte Gel Collapse and Reentrant Swelling. Macromolecules 2013, 46, 5053–5065. [Google Scholar] [CrossRef]
- Siirilä, J.; Häkkinen, S.; Tenhu, H. The emulsion polymerization induced self-assembly of a thermoresponsive polymer poly(N-vinylcaprolactam. Polym. Chem. 2019, 10, 766–775. [Google Scholar] [CrossRef]
- Burchard, W. Static and dynamic light scattering from branched polymers and biopolymers. Adv. Polym. Sci. 1998, 48, 1–124. [Google Scholar]
- Rovigatti, L.; Gnan, N.; Tavagnacco, L.; Moreno, A.J.; Zaccarelli, E. Numerical modelling of non-ionic microgels: An overview. Soft Matter 2019, 15, 1108–1119. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, T.-W. Electrophoretic Mobility, Zeta Potential, and Fixed Charge Density of Bovine Knee Chondrocytes, Methyl Methacrylate−Sulfopropyl Methacrylate, Polybutylcyanoacrylate, and Solid Lipid Nanoparticles. J. Phys. Chem. B 2006, 110, 2202–2208. [Google Scholar] [CrossRef]
- Lee, S.-P.; Duong, T.Q.; Yang, G.; Iadecola, C.; Kim, S.-G. Relave changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: Implications for BOLD fMRI. Magn. Reson. Med. 2001, 45, 791–800. [Google Scholar] [CrossRef]
- Whang, C.J.; Monzingo, J.R.; Rhoton, A.L. Comparison of Blood Flow and Patency in Arterial and Vein Grafts to Basilar Artery. Stroke 1975, 6, 445–448. [Google Scholar] [CrossRef]
- Ashley, N.; Poulton, J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem. Biophys. Res. Commun. 2009, 378, 450–455. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Spiridonov, V.V.; Lukmanova, A.R.; Pozdyshev, D.V.; Markova, A.A.; Volodina, Y.V.; Golovina, G.V.; Shakhmatov, V.V.; Kuzmin, V.A.; Muronetz, V.I.; Yaroslavov, A.A. Ionically cross-linked micro-sized hydrogels with encapsulated drug: Structure, cell uptake kinetics and cytotoxicity. Mendeleev Comm. 2023, 33, 553–555. [Google Scholar] [CrossRef]
- Anand, R.; Ottani, S.; Manoli, F.; Manet, I.; Monti, S. A close-up on doxorubicin binding to γ-cyclodextrin: An elucidating spectroscopic, photophysical and conformational study. RSC Adv. 2012, 2, 2346. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xiong, W.; Sun, S.; Zhang, P.; Zhu, J.J. Recent advances in drug release monitoring. Nanophotonics 2019, 8, 391–413. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]











| No | [HYAL]/[Fe2+] Ratio in Reaction Mixture | η, wt% |
|---|---|---|
| I | 4/1 | 5.12 |
| II | 2/1 | 14.0 |
| III | 1/1 | 17.0 |
| IV | 1/2 | 26.8 |
| Sample | Fe Content, wt% | Dh in Bi-Distilled Water, nm | Dh in 0.15 M NaCl Aqueous Solution, nm | EPM in pH 7.0 TRIS Buffer, (μm/s)/(V/cm) |
|---|---|---|---|---|
| HYAL | 0 | 275 | 160 | −4.23 |
| I | 5.12 | 265 | 126 | −3.21 |
| II | 14.0 | 225 | 130 | −3.21 |
| III | 17.9 | 200 | 111 | −2.85 |
| IV | 26.8 | 150 | 110 | −1.68 |
| Sample | Fe Content, wt% | Mw × 10−6, Da | Rg, nm | Rh, nm | Rg/Rh | 2A2 × 10−5, Mole cm3 g−2 |
|---|---|---|---|---|---|---|
| I | 5.12 | 100 | 79 | 180 | 0.44 | 1.35 |
| II | 14.0 | 180 | 67 | 150 | 0.45 | 1.18 |
| III | 17.0 | 220 | 59 | 125 | 0.47 | 1.15 |
| IV | 26.8 | 250 | 55 | 85 | 0.64 | 0.9 |
| № | HYAL/PA Ratio in Reaction Mixture, wt/wt Ratio | η, wt% |
|---|---|---|
| Ipa | 10/0.5 | 15.8 |
| IIpa | 10/1 | 17.2 |
| IIIpa | 10/2 | 36.4 |
| IVpa | 10/3 | 38.0 |
| Magnetostatic Characteristic | Binary Composites | Ternary Composites | ||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | Ipa | IIpa | IIIpa | IVpa | |
| Is, emu/g | 0.15 | 0.25 | 0.6 | 1.1 | 0.3 | 0.31 | 1.48 | 1.84 |
| Ir, emu/g | 0.05 | 0.06 | 0.06 | 0.06 | 0.01 | 0.03 | 0.25 | 0.29 |
| Hc, Э | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | 0.5 | 0.5 |
| Flow Rate, cm/s | Composite IV | Composite IVpa |
|---|---|---|
| 0.17 | + | + |
| 0.37 | + | + |
| 0.62 | − | + |
| 1.37 | − | + |
| 1.84 | − | + |
| Sample | IC50, μM | |
|---|---|---|
| Carboxylic Groups | Dox | |
| III | >1000 | n/a |
| IIpa | >1000 | n/a |
| Dox | n/a | 1.06 ± 0.05 |
| III-Dox | n/a | 1.03 ± 0.04 |
| IIpa-Dox | n/a | 1.05 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridonov, V.; Zoirova, Z.; Alyokhina, Y.; Perov, N.; Afanasov, M.; Pozdyshev, D.; Krjukova, D.; Knotko, A.; Muronetz, V.; Yaroslavov, A. Magnetically Controlled Hyaluronic Acid–Maghemite Nanocomposites with Embedded Doxorubicin. Polymers 2023, 15, 3644. https://doi.org/10.3390/polym15173644
Spiridonov V, Zoirova Z, Alyokhina Y, Perov N, Afanasov M, Pozdyshev D, Krjukova D, Knotko A, Muronetz V, Yaroslavov A. Magnetically Controlled Hyaluronic Acid–Maghemite Nanocomposites with Embedded Doxorubicin. Polymers. 2023; 15(17):3644. https://doi.org/10.3390/polym15173644
Chicago/Turabian StyleSpiridonov, Vasily, Zukhra Zoirova, Yuliya Alyokhina, Nikolai Perov, Mikhail Afanasov, Denis Pozdyshev, Daria Krjukova, Alexander Knotko, Vladimir Muronetz, and Alexander Yaroslavov. 2023. "Magnetically Controlled Hyaluronic Acid–Maghemite Nanocomposites with Embedded Doxorubicin" Polymers 15, no. 17: 3644. https://doi.org/10.3390/polym15173644
APA StyleSpiridonov, V., Zoirova, Z., Alyokhina, Y., Perov, N., Afanasov, M., Pozdyshev, D., Krjukova, D., Knotko, A., Muronetz, V., & Yaroslavov, A. (2023). Magnetically Controlled Hyaluronic Acid–Maghemite Nanocomposites with Embedded Doxorubicin. Polymers, 15(17), 3644. https://doi.org/10.3390/polym15173644