Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition
Abstract
:1. Introduction
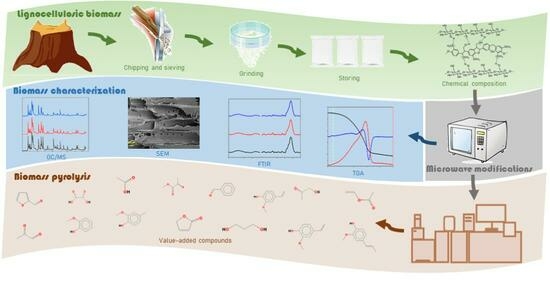
2. Materials and Methods
2.1. Raw Material
2.2. Methods
2.2.1. Biomass before Pretreatment Characterisation
Proximate, Ultimate Analysis and Calorific Value
Chemical Composition
2.2.2. Microwave Irradiation
2.2.3. Biomass Pretreated Characterisation
Thermogravimetric Analysis (TGA)
Fourier Transform Infrared (FTIR) Spectroscopy
Morphology Analysis
2.2.4. Extractive Characterisation
2.2.5. Pyrolysis Analysis
3. Results
3.1. Biomass Untreated Characterisation
Proximate, Ultimate Analysis, Calorific Value, and Chemical Composition
3.2. Microwave Pretreatment
3.3. Effect of MW on Biomass Chemical Composition
3.4. Biomass Pretreated Characterisation
3.4.1. Thermal and Kinetic Parameters
Thermal Decomposition Characteristics and Heating Rate Effects
Activation Energy (Ea) Using Isoconversional Models
3.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.4.3. Surface Morphology
3.4.4. Extractives Characterisation
3.5. Py–GC/MS Analysis
3.5.1. Family Compounds Formed during PR and EG Pyrolysis
3.5.2. Chemical Compounds Formed during PR and EG Pyrolysis
3.5.3. Reaction Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafizadeh, A.; Rastegari, H.; Shahbeik, H.; Mobli, H.; Pan, J.; Peng, W.; Li, G.; Tabatabaei, M.; Aghbashlo, M. A Critical Review of the Use of Nanomaterials in the Biomass Pyrolysis Process. J. Clean. Prod. 2023, 400, 136705. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2022, 8, 10–114. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima Devi, T.; Sivashanmugam, P.; Kavitha, S.; Yukesh Kannah, R.; Varjani, S.; AdishKumar, S.; Kumar, G.; Rajesh Banu, J. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Korus, A.; Szlęk, A.; Samson, A. Physicochemical Properties of Biochars Prepared from Raw and Acetone-Extracted Pine Wood. Fuel Process. Technol. 2019, 185, 106–116. [Google Scholar] [CrossRef]
- Álvarez González, V.; Poblete Hernández, P.; Soto Aguirre, D.; Caselli, J.G.; Kahler González, C.; Pardo Velásquez, E.; Carlos, J.; Munita, B.; Rocha, D.B. Anuario Forestal Chilean Statistical Yearbook of Forestry 2022; Anuario Forestal Infor: La Serena, Chile, 2022; Volume 187, pp. 1–280. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.; Segura, C.; Diéguez, K. Procesos de Torrefacción Para Valorización de Residuos Lignocelulósicos. Análisis Posibles Tecnol. Apl. Sudamérica Afinidad LXXIII 2016, 573, 60–68. [Google Scholar]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose Biomass Pyrolysis for Bio-Oil Production: A Review of Biomass Pretreatment Methods for Production of Drop-in Fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various Pretreatments of Lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A Review of Wood Thermal Pretreatments to Improve Wood Composite Properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Deivayanai, V.C.; Yaashikaa, P.R.; Senthil Kumar, P.; Rangasamy, G. A Comprehensive Review on the Biological Conversion of Lignocellulosic Biomass into Hydrogen: Pretreatment Strategy, Technology Advances and Perspectives. Bioresour. Technol. 2022, 365, 128166. [Google Scholar] [CrossRef]
- Sunarti, T.C.; Dwiko, M.; Derosya, V.; Meryandini, A. Effect of Microwave Treatment on Acid and Enzymes Susceptibilities of Sago Pith. Procedia Chem. 2012, 4, 301–307. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of Lignocellulosic Wastes for Biofuel Production: A Critical Review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Fia, A.Z.; Amorim, J. Microwave Pretreatment of Biomass for Conversion of Lignocellulosic Materials into Renewable Biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Ma Rodríguez-Jasso, R.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave Heating Processing as Alternative of Pretreatment in Second-Generation Biorefinery: An Overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Luo, K.; Shao, J.; Yang, H. The Influence of Microwave Drying on Biomass Pyrolysis. Energy Fuels 2008, 22, 67–74. [Google Scholar] [CrossRef]
- Gao, H.; Bai, J.; Wei, Y.; Chen, W.; Li, L.; Huang, G.; Li, P.; Chang, C. Effects of Drying Pretreatment on Microwave Pyrolysis Characteristics of Tobacco Stems. Biomass Convers. Biorefin. 2022, 13, 11521–11531. [Google Scholar] [CrossRef]
- Cruz, N.; Bustos, C.; Aguayo, M.G.; Cloutier, A.; Castillo, R. THM Densification of Wood. Bioresources 2018, 13, 2268–2282. [Google Scholar]
- ASTM D5373-08; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal 2002. ASTM: West Conshohocken, PA, USA, 2002.
- ASTM D3172; Standard Practice for Proximate Analysis of Coal and Coke. ASTM: West Conshohocken, PA, USA, 2013.
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- TAPPI T280 pm-99 (2000); Acetone extractives of wood and pulp. Technical Association of the Pulp and Paper Industry, TAPPI Press: Norcross, GA, USA, 2000.
- TAPPI T9 wd-75 (2015); Holocellulose in wood. Technical Association of the Pulp and Paper Industry, TAPPI Press: Norcross, GA, USA, 2015.
- TAPPI T222 om-11 (2015); Acid-insoluble lignin in wood and pulp. Technical Association of the Pulp and Paper Industry, TAPPI Press: Norcross, GA, USA, 2015.
- Dence, C.W. The determination of lignin. In Methods in Lignin Chemistry; Springer-Verlag: Berlin/Heidelberg, Germany, 1992; Volume 2, pp. 33–61. [Google Scholar]
- Venegas, D.; Alejandro-Martín, S. Influencia Del Pretratamiento Con Microondas En La Pirólisis Analítica de Biomasa Lignocelulósica. In Proceedings of the Encuentro Iberoamericano de Redes de Biomasa y Bioenergía, Virtual, 13–15 October 2021; pp. 103–108. [Google Scholar]
- Collazzo, G.C.; Broetto, C.C.; Perondi, D.; Junges, J.; Dettmer, A.; Dornelles Filho, A.A.; Foletto, E.L.; Godinho, M. A Detailed Non-Isothermal Kinetic Study of Elephant Grass Pyrolysis from Different Models. Appl. Therm. Eng. 2017, 110, 1200–1211. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. Part. C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.; Wall, L. General Treatment of the Thermogravimety of Polymers. J. Res. Natl. Bur. Standars 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analysing Thermogravimetric Data. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural characteristics and thermal properties of native cellulose. In Cellulose—Fundamental Aspects; InTech: Houston, TX, USA, 2013. [Google Scholar]
- Fabbri, D.; Chiavari, G.; Prati, S.; Vassura, I.; Vangelista, M. Gas Chromatography/Mass Spectrometric Characterisation of Pyrolysis/Silylation Products of Glucose and Cellulose. Rapid Commun. Mass. Spectrom. 2002, 16, 2349–2355. [Google Scholar] [CrossRef]
- Niu, Q.; Ghysels, S.; Wu, N.; Rousseau, D.P.L.; Pieters, J.; Prins, W.; Ronsse, F. Effects of Demineralization on the Composition of Microalgae Pyrolysis Volatiles in Py-GC–MS. Energy Convers. Manag. 2022, 251, 114979. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Segura, C.; Espinoza, D.; Radovic, L.R.; Jiménez, R. Torrefaction of Pinus Radiata and Eucalyptus Globulus: A Combined Experimental and Modeling Approach to Process Synthesis. Energy Sustain. Dev. 2015, 29, 13–23. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Chi-Wei Lan, J.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.S.; Allakhverdiev, S.I. A Comprehensive Review on Lignocellulosic Biomass Biorefinery for Sustainable Biofuel Production. Int. J. Hydrog. Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Campañone, L.A.; Paola, C.A.; Mascheroni, R.H. Modeling and Simulation of Microwave Heating of Foods Under Different Process Schedules. Food Bioproc. Tech. 2012, 5, 738–749. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q.; Ma, C.; Zhang, J.; Zhao, X.; Song, Z. Review on Microwave-Metal Discharges and Their Applications in Energy and Industrial Processes. Appl. Energy 2016, 175, 141–157. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Tsubaki, S.; Azuma, J.-I. Application of Microwave Technology for Utilization of Recalcitrant Biomass. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; IntechOpen: London, UK, 2011; pp. 697–722. [Google Scholar] [CrossRef]
- Liu, H.; Shang, J.; Chen, X.; Kamke, F.A.; Guo, K. The Influence of Thermal-Hydro-Mechanical Processing on Chemical Characterization of Tsuga Heterophylla. Wood Sci. Technol. 2014, 48, 373–392. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Wu, F. Chemical Analysis of Densification, Drying, and Heat Treatment of Scots Pine (Pinus sylvestris L.) through a Hot-Pressing Process. Bioresources 2016, 11, 3856–3874. [Google Scholar] [CrossRef]
- Correa, L.; Hernández, E. El Uso de Las Microondas En La Industria Farmacéutica. Rev. Mex. Cienc. Farm. 2011, 42, 6–25. [Google Scholar]
- Savitzky, A.; Golay, M. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Cerda-Barrera, C.; Fernández-Andrade, K.J.; Alejandro-Martín, S. Pyrolysis of Chilean Southern Lignocellulosic Biomasses: Isoconversional Kinetics Analysis and Pyrolytic Products Distribution. Polymers 2023, 15, 2698. [Google Scholar] [CrossRef] [PubMed]
- Grønli, M.G.; Várhegyi, G.; Di Blasi, C. Thermogravimetric Analysis and Devolatilization Kinetics of Wood. Ind. Eng. Chem. Res. 2002, 41, 4201–4208. [Google Scholar] [CrossRef]
- Shebani, A.N.; van Reenen, A.J.; Meincken, M. The Effect of Wood Extractives on the Thermal Stability of Different Wood-LLDPE Composites. Thermochim. Acta 2009, 481, 52–56. [Google Scholar] [CrossRef]
- Antal, M.J.; Varhegyi, G. Cellulose Pyrolysis Kinetic: The Current State of Knowledge. Ind. Eng. Chem. Res. 1995, 34, 703–717. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Kinetic Analysis and Pyrolysis Behaviour of Waste Biomass towards Its Bioenergy Potential. Bioresour. Technol. 2020, 311, 123480. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR Study of Pea Waste Biomass Pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Bartocci, P.; Tschentscher, R.; Stensrød, R.E.; Barbanera, M.; Fantozzi, F. Kinetic Analysis of Digestate Slow Pyrolysis with the Application of the Master-Plots Method and Independent Parallel Reactions Scheme. Molecules 2019, 24, 1657. [Google Scholar] [CrossRef]
- de Carvalho, V.S.; Tannous, K. Thermal Decomposition Kinetics Modeling of Energy Cane Saccharum Robustum. Thermochim. Acta 2017, 657, 56–65. [Google Scholar] [CrossRef]
- Guo, X.J.; Wang, S.R.; Wang, K.G.; Liu, Q.; Luo, Z.Y. Influence of the Extractives on Mechanism of Biomass Pyrolysis. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2010, 38, 42–46. [Google Scholar] [CrossRef]
- Binner, J.G.P.; Hassine, N.A.; Cross, T.E. The Possible Role of the Pre-Exponential Factor in Explaining the Increased Reaction Rates Observed during the Microwave Synthesis of Titanium Carbide. J. Mater. Sci. 1995, 30, 5389–5393. [Google Scholar] [CrossRef]
- Dong, Q.; Xiong, Y. Kinetics Study on Conventional and Microwave Pyrolysis of Moso Bamboo. Bioresour. Technol. 2014, 171, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Henrique, P.; Pereira, F.; Fernandes, L.; Benini, K.; Pereira, B.; Ornaghi, H.; Cioo, M.O. Different Sequential Chemical Treatments Used to Obtain Bleached Cellulose from Orange Bagasse. J. Nat. Fibers 2022, 19, 12849–12861. [Google Scholar] [CrossRef]
- Carrillo, I.; Mendonça, R.T.; Ago, M.; Rojas, O.J. Comparative Study of Cellulosic Components Isolated from Different Eucalyptus Species. Cellulose 2018, 25, 1011–1029. [Google Scholar] [CrossRef]
- Dai, L.; He, C.; Wang, Y.; Liu, Y.; Yu, Z.; Zhou, Y.; Fan, L.; Duan, D.; Ruan, R. Comparative Study on Microwave and Conventional Hydrothermal Pretreatment of Bamboo Sawdust: Hydrochar Properties and Its Pyrolysis Behaviors. Energy Convers. Manag. 2017, 146, 1–7. [Google Scholar] [CrossRef]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Chemical Changes of Heat Treated Pine and Eucalypt Wood Monitored by Ftir. Maderas Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef]
- Gu, X.; Ma, X.; Li, L.; Liu, C.; Cheng, K.; Li, Z. Pyrolysis of Poplar Wood Sawdust by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Pandey, K.K. A Study of Chemical Structure of Soft and Hardwood and Wood Polymers by FTIR Spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef]
- Jahan, M.S.; Chowdhury, D.A.N.; Islam, M.K.; Moeiz, S.M.I. Characterisation of Lignin Isolated from Some Nonwood Available in Bangladesh. Bioresour. Technol. 2007, 98, 465–469. [Google Scholar] [CrossRef]
- Dubey, M. Improvements in Stability, Durability and Mechanical Properties of Radiata Pine Wood after Heat-Treatment in a Vegetable Oil. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2010. [Google Scholar]
- Wang, Z.; Yao, Z.J.; Zhou, J.; Zhang, Y. Reuse of Waste Cotton Cloth for the Extraction of Cellulose Nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation and Characterization of Nanocellulose from Beer Industrial Residues Using Acid Hydrolysis/Ultrasound. Fibers Polym. 2015, 16, 529–536. [Google Scholar] [CrossRef]
- Tajmirriahi, M.; Karimi, K.; Kumar, R. Effects of Pinewood Extractives on Bioconversion of Pinewood. Fuel 2021, 283, 119302. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Yu, Z.; Chen, L.; Liao, Y.; Ma, X. Effects of Microwave Pretreatment on Catalytic Fast Pyrolysis of Pine Sawdust. Bioresour. Technol. 2019, 293, 122080. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of Microwave and NaOH Pretreatments of Wheat Straw for Enhancing Biofuel Yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the Recent Advances of Microwave Pretreatment Technologies for the Conversion of Lignocellulosic Biomass into Sustainable Biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef] [PubMed]
- de Paula Protásio, T.; Lima, M.D.R.; Teixeira, R.A.C.; do Rosário, F.S.; de Araújo, A.C.C.; de Assis, M.R.; Hein, P.R.G.; Trugilho, P.F. Influence of Extractives Content and Lignin Quality of Eucalyptus Wood in the Mass Balance of Pyrolysis Process. Bioenergy Res. 2021, 14, 175–189. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Gutiérrez, A.; Martín, F.; Verdejo, T. Application of Analytical Pyrolysis to the Characterization of Eucalyptus Extractives and Pitch Deposits from a Pulp Mill. J. Anal. Appl. Pyrolysis 1997, 40–41, 501–510. [Google Scholar] [CrossRef]
- Park, C.; Sheehan, R.J. Phthalic acids and other benzenepolycarboxylic acids. In Kirk-Othmer Encyclopedia of Chemical Technology; WILEY: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Mészáros, E.; Jakab, E.; Várhegyi, G. TG/MS, Py-GC/MS and THM-GC/MS Study of the Composition and Thermal Behavior of Extractive Components of Robinia Pseudoacacia. J. Anal. Appl. Pyrolysis 2007, 79, 61–70. [Google Scholar] [CrossRef]
- Wang, H.; Male, J.; Wang, Y. Recent Advances in Hydrotreating of Pyrolysis Bio-Oil and Its Oxygen-Containing Model Compounds. ACS Catal. 2013, 3, 1047–1070. [Google Scholar] [CrossRef]
- Wentzel, M.; Rolleri, A.; Pesenti, H.; Militz, H. Chemical Analysis and Cellulose Crystallinity of Thermally Modified Eucalyptus Nitens Wood from Open and Closed Reactor Systems Using FTIR and X-Ray Crystallography. Eur. J. Wood Wood Prod. 2019, 77, 517–525. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Usino, D.O.; Ylitervo, P.; Moreno, A.; Sipponen, M.H.; Richards, T. Primary Interactions of Biomass Components during Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2021, 159, 105297. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Veeranki, V.D.; Goud, V.V. Lignocellulosic Feedstocks for the Production of Bioethanol: Availability, Structure, and Composition; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128176542. [Google Scholar]
- Piskorz, J.; Radlein, D.S.A.G.; Scott, D.S.; Czernik, S. Pretreatment of Wood and Cellulose for Production of Sugars by Fast Pyrolysis. J. Anal. Appl. Pyrolysis 1989, 16, 127–142. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Hunt, A.J.; Budarin, V.L.; Attard, T.M.; Kling, J.; Surup, G.R.; Arshadi, M.; Umeki, K. Supercritical Extraction and Microwave Activation of Wood Wastes for Enhanced Syngas Production and Generation of Fullerene-like Soot Particles. Fuel Process. Technol. 2021, 212, 106633. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, G.G. Pyrolysis of Lignocellulosic Biomass for Biochemical Production; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444639929. [Google Scholar]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into Biomass Pyrolysis Mechanism Based on Cellulose, Hemicellulose, and Lignin: Evolution of Volatiles and Kinetics, Elucidation of Reaction Pathways, and Characterization of Gas, Biochar and Bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. Biofuel Production and Kinetics Analysis for Microwave Pyrolysis of Douglas Fir Sawdust Pellet. J. Anal. Appl. Pyrolysis 2012, 94, 163–169. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Brown, R.C.; Shanks, B.H. Product Distribution from the Fast Pyrolysis of Hemicellulose. ChemSusChem 2011, 4, 636–643. [Google Scholar] [CrossRef]
- Yang, H.; Gong, M.; Chen, W.; Fang, Y.; Chen, Y.; Wang, X.; Chen, H. Lignin Pyrolysis under NH3 Atmosphere for 4-Vinylphenol Product: An Experimental and Theoretical Study. Fuel 2021, 297, 120776. [Google Scholar] [CrossRef]
- Ma, H.; Liu, W.W.; Chen, X.; Wu, Y.J.; Yu, Z.L. Enhanced Enzymatic Saccharification of Rice Straw by Microwave Pretreatment. Bioresour. Technol. 2009, 100, 1279–1284. [Google Scholar] [CrossRef] [PubMed]





| Method | Expression | Plots | Ref. |
|---|---|---|---|
| Friedmann | ln (dα/dt) = ln [A·f(α)] − Ea/R·T | ln (dα/dt) vs. 1/T | [31] |
| Flynn-Wall-Ozawa | ln β = ln [A·Ea/R·g(α)] − 5.3305 − 1.052 Ea/R·T | ln β vs. 1/T | [32,33] |
| Coats-Redfern | ln [β/T2 (1 − 2R·T/Ea))] = ln [−A·R/Ea·ln(1 − α))] − Ea/R·T | ln (β/T2) vs. 1/T | [34] |
| Proximate Analysis | Ultimate Analysis | Chemical Composition | ||||||
|---|---|---|---|---|---|---|---|---|
| PR | EG | PR | EG | PR | EG | |||
| Moisture (%) | 7.75 | 5.51 | Carbon (%) | 48.02 | 47.76 | Hemicellulose (%) | 28.6 ± 0.8 | 26.3 ± 0.3 |
| Volatiles (%) | 76.73 | 77.13 | Hydrogen (%) | 5.90 | 6.32 | Cellulose (%) | 43.1 ± 0.1 | 53.0 ± 0.2 |
| Fixed carbon (%) | 14.68 | 16.85 | Nitrogen (%) | 0.29 | 0.09 | Lignin (%) | 26.6 ± 1.6 | 23.9 ± 2.1 |
| Ash (%) | 0.83 | 0.51 | Sulphur (%) | 0.10 | 0.05 | Extractives (%) | 1.8 ± 0.3 | 1.9 ± 0.0 |
| HHV (MJ/kg) | 18.98 | 19.38 | Oxygen (%) | 45.69 | 45.77 | |||
| Samples | Pretreatment Condition | Hemicellulose (%) | Δ * (%) | Cellulose (%) | Δ * (%) | Lignin (%) | Δ * (%) | Extractives (%) | Δ * (%) |
|---|---|---|---|---|---|---|---|---|---|
| PR | 259 W-5 min | 29.3 ± 1.5 | −0.7 | 41.0 ± 0.3 | 2.1 | 24.5 ± 1.3 | 2.1 | 3.0 ± 0.0 | −1.2 |
| 700 W-5 min | 28.8 ± 0.7 | −0.2 | 41.3 ± 0.4 | 1.8 | 24.4 ± 1.5 | 2.2 | 7.2 ± 0.1 | −5.4 | |
| EG | 259 W-5 min | 28.0 ± 0.5 | −1.7 | 50.0 ± 0.3 | 3.0 | 22.8 ± 1.7 | 1.1 | 3.8 ± 0.4 | −1.9 |
| 700 W-5 min | 31.2 ± 0.2 | −4.9 | 50.4 ± 0.2 | 2.6 | 22.3 ± 0.5 | 1.6 | 6.5 ± 0.2 | −4.6 |
| Biomass | Microwave Pretreatment | FR Method | FWO Method | Coats–Redfern Method | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea | A | R2 | Ea | A | R2 | Ea | A | R2 | ||
| kJ·mol−1 | min−1 | kJ·mol−1 | min−1 | kJ·mol−1 | min−1 | |||||
| WOT | 183.03 | 30.48 | 0.9898 | 201.71 | 39.23 | 0.9800 | 202.05 | 38.38 | 0.9780 | |
| PR | 259 | 177.95 | 33.08 | 0.9956 | 185.07 | 40.83 | 0.9962 | 185.04 | 40.34 | 0.9967 |
| 700 | 164.69 | 34.21 | 0.9973 | 174.91 | 45.01 | 0.9978 | 175.01 | 43.94 | 0.9971 | |
| WOT | 156.19 | 26.48 | 0.9956 | 174.80 | 34.28 | 0.9989 | 165.89 | 33.65 | 0.9990 | |
| EG | 259 | 150.42 | 27.94 | 0.9911 | 166.72 | 35.94 | 0.9946 | 158.28 | 35.26 | 0.9938 |
| 700 | 143.30 | 29.10 | 0.9889 | 158.51 | 37.57 | 0.9910 | 150.38 | 36.79 | 0.9898 | |
| Sample | Pretreatment | Crystallinity Index |
|---|---|---|
| PR | WOT | 1.31 |
| 259-5 | 1.04 | |
| 700-5 | 0.82 | |
| EG | WOT | 0.81 |
| 259-5 | 0.68 | |
| 700-5 | 0.67 |
| Chemical Compounds | PR %Area | EG %Area | ||||
|---|---|---|---|---|---|---|
| WOT | 259 | 700 | WOT | 259 | 700 | |
| 1,2-Benzenedicarboxylic acid | 73.67 | 49.67 | 4.17 | 15.52 | 23.08 | 15.82 |
| 2-Propenoic acid | 5.57 | 1.90 | n.d | 6.39 | n.d | 1.11 |
| Acetic acid | 4.18 | 8.47 | 2.27 | 43.05 | 4.02 | 24.14 |
| Oxalic acid, allyl nonyl ester | 2.09 | 11.29 | 45.02 | 4.70 | 15.72 | 20.54 |
| Acetic acid, chloro-, methyl ester | n.d | 0.00 | 16.55 | 12.40 | 6.48 | 6.45 |
| Butanal, 3-methyl- | n.d | 1.74 | 11.09 | 0.00 | 17.73 | 5.26 |
| Acetaldehyde | n.d | 2.48 | 12.97 | n.d | n.d | 10.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venegas-Vásconez, D.; Arteaga-Pérez, L.E.; Aguayo, M.G.; Romero-Carrillo, R.; Guerrero, V.H.; Tipanluisa-Sarchi, L.; Alejandro-Martín, S. Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition. Polymers 2023, 15, 3790. https://doi.org/10.3390/polym15183790
Venegas-Vásconez D, Arteaga-Pérez LE, Aguayo MG, Romero-Carrillo R, Guerrero VH, Tipanluisa-Sarchi L, Alejandro-Martín S. Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition. Polymers. 2023; 15(18):3790. https://doi.org/10.3390/polym15183790
Chicago/Turabian StyleVenegas-Vásconez, Diego, Luis E. Arteaga-Pérez, María Graciela Aguayo, Romina Romero-Carrillo, Víctor H. Guerrero, Luis Tipanluisa-Sarchi, and Serguei Alejandro-Martín. 2023. "Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition" Polymers 15, no. 18: 3790. https://doi.org/10.3390/polym15183790
APA StyleVenegas-Vásconez, D., Arteaga-Pérez, L. E., Aguayo, M. G., Romero-Carrillo, R., Guerrero, V. H., Tipanluisa-Sarchi, L., & Alejandro-Martín, S. (2023). Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition. Polymers, 15(18), 3790. https://doi.org/10.3390/polym15183790