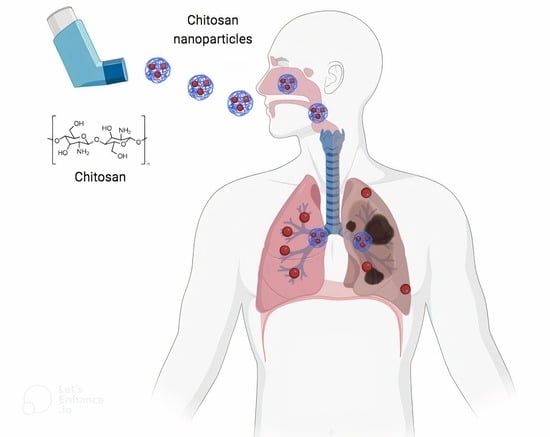
Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery
Abstract
:1. Introduction
2. Pulmonary Route: Challenges and Opportunities
3. Chitosan: Physicochemical and Biological Properties
4. Preparation of Chitosan-Based Nanoparticles
5. Chitosan-Based Nanoparticles for Pulmonary Delivery
5.1. Pulmonary Chronic Diseases
5.1.1. Asthma
5.1.2. Chronic Obstructive Pulmonary Disease (COPD)
5.1.3. Pulmonary Fibrosis
5.1.4. Idiopathic Pulmonary Fibrosis (IPF)
5.1.5. Cystic Fibrosis
5.2. Lung Cancer
5.3. Infectious Diseases
5.3.1. Tuberculosis
5.3.2. Pneumonia
5.3.3. COVID-19
6. Patents
| Patent Name | Patent Number | Country | Type | Chitosan Function | Disease | Active Pharmaceutical Ingredient Type | Ref. |
|---|---|---|---|---|---|---|---|
| Gsk3 inhibitor-loaded nano formulations as a cancer immunotherapeutic | WO2022006083A1 | US | Lipid-based | Drug carrier | Cancer | GSK3 inhibitor | [161] |
| Novel method for dry powder inhalation comprising. | AU2014204483A1 | AU | Lipidic | Enhance retention in lung tissue | Lung tissues diseases | not specified | [165] |
| Quercetin and paclitaxel co-transportation pulmonary-inhaled nanometer-targeted porous polymer particle and preparation method thereof | CN106309411A | CN | Polymeric | Formulation Ingredient | Lung cancer | Quercetin and paclitaxel | [166] |
| A pulmonary-inhaled chitosan-based nano-targeting polymer particles and its production method thereof | CN106265607A | CN | Polymeric | Enhancer of bioavailability and stability | Cancer | Monoclonal antibody cetuximab | [167] |
| Nano-delivery system for inhaled chemotherapy | WO2022119528A1 | TR | Polymeric | Enhance retention in lung tissue | Lung cancer | Doxorubicin | [168] |
| Method of use for Apoe peptides | WO2023288316A1 | US | Lipid-based | Targeting | Miscellaneous | Organic molecules, nucleic acid, peptides, and protein | [162] |
| Therapeutic methods and compositions comprising magnetizable nanoparticles | WO2022187556A1 | US | Magnetic | Surface functionalization | Miscellaneous | Peptides, polymers, contrasting agents, imaging agents, and combinations thereof | [169] |
| Immunotherapeutic constructs and methods of their use | WO2021011496A1 | US | Lipid-based, polymeric, and inorganic | Surface functionalization | Cancer | Antibody, nucleic acid, oligonucleotides, and small molecules | [163] |
| Hollow particles encapsulating a biological gas and methods of use | WO2014143808A1 | US | Polymeric | Surface functionalization | Local or systemic hypoxia | Therapeutic gasses | [170] |
| Npc1 monobodies and monobody conjugates thereof | WO2022103840A2 | US | Polymeric or lipid-based | Formulation Ingredient | Niemann-Pick disease | Peptides | [164] |
| Pd-l1-binding peptides and peptide complexes and methods of use thereof | WO2022115719A1 | US | Not specified | Permeation enhancer | Cancer | Peptides | [171] |
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56. [Google Scholar] [CrossRef]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.C.; Tan, P.S.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021, 9, 909–923. [Google Scholar] [CrossRef]
- Fei, Q.; Bentley, I.; Ghadiali, S.N.; Englert, J.A. Pulmonary drug delivery for acute respiratory distress syndrome. Pulm. Pharmacol. Ther. 2023, 79, 102196. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Newman, S. Improving inhaler technique, adherence to therapy and the precision of dosing: Major challenges for pulmonary drug delivery. Expert Opin. Drug. Deliv. 2014, 11, 365–378. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, Y.; Han, R.; Lu, W.L.; Mak, J.C.W.; Zheng, Y. Rational particle design to overcome pulmonary barriers for obstructive lung diseases therapy. J. Control. Release 2019, 314, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.K.; Nichols, B.L.B.; Edgar, K.J.; Murgia, X.; Loretz, B.; Lehr, C.M. Challenges and strategies in drug delivery systems for treatment of pulmonary infections. Eur. J. Pharm. Biopharm. 2019, 144, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Chellappan, D.K.; Dua, K.; Mehta, M.; Satija, S.; Singh, I. Patented therapeutic drug delivery strategies for targeting pulmonary diseases. Expert Opin. Ther. Pat. 2020, 30, 375–387. [Google Scholar] [CrossRef]
- Kole, E.; Jadhav, K.; Shirsath, N.; Dudhe, P.; Verma, R.K.; Chatterjee, A.; Naik, J. Nanotherapeutics for pulmonary drug delivery: An emerging approach to overcome respiratory diseases. J. Drug Deliv. Sci. Technol. 2023, 81, 104261. [Google Scholar] [CrossRef]
- Lim, Y.H.; Tiemann, K.M.; Hunstad, D.A.; Ellsabahy, M.; Wooley, K.L. Polymeric nanoparticles in development for treatment of pulmonary infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 842–871. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Shiehzadeh, F.; Tafaghodi, M. Dry Powder form of Polymeric Nanoparticles for Pulmonary Drug Delivery. Curr. Pharm. Des. 2016, 22, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Rasul, R.M.; Tamilarasi Muniandy, M.; Zakaria, Z.; Shah, K.; Chee, C.F.; Dabbagh, A.; Rahman, N.A.; Wong, T.W. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr. Polym. 2020, 250, 116800. [Google Scholar] [CrossRef] [PubMed]
- Rawal, T.; Patel, S.; Butani, S. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur. J. Pharm. Sci. 2018, 124, 283–287. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Weifen, Z. Applications of Chitosan in Pulmonary Drug Delivery. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; IntechOpen: Rijeka, Croatia, 2020; Applications of Chitosan in Pulmonary Drug Delivery. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 11995. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Almakhamel, M.Z.; Ansari, K.; Amir, M.; Ahmad, W.; Ali, A.; Ahmad, F.J. A comparative pulmonary pharmacokinetic study of budenoside using polymeric nanoparticles targeted to the lungs in treatment of asthma. Artif. Cells Nanomed. Biotechnol. 2020, 48, 749–762. [Google Scholar] [CrossRef]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Singh, S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef]
- Hill, M.; Twigg, M.; Sheridan, E.A.; Hardy, J.G.; Elborn, J.S.; Taggart, C.C.; Scott, C.J.; Migaud, M.E. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics 2019, 11, 379. [Google Scholar] [CrossRef]
- Kamel, R.; Deeb, N.M.; Abbas, H. Development of a potential anti-cancer pulmonary nanosystem consisted of chitosan-doped LeciPlex loaded with resveratrol using a machine learning method. J. Drug Deliv. Sci. Technol. 2022, 70, 103259. [Google Scholar] [CrossRef]
- Tavakol, S.; Zahmatkeshan, M.; Mohammadinejad, R.; Mehrzadi, S.; Joghataei, M.T.; Alavijeh, M.S.; Seifalian, A. The role of nanotechnology in current COVID-19 outbreak. Heliyon 2021, 7, e06841. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.D.L.; Cajubá de Britto Lira Nogueira, M. Pharmaceutical nanotechnology: Which products are been designed against COVID-19? J. Nanopart. Res. 2020, 22, 276. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Haji Molla Hoseini, M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Chablani, L. A comprehensive overview of dry powder inhalers for pulmonary drug delivery: Challenges, advances, optimization techniques, and applications. J. Drug Deliv. Sci. Technol. 2023, 84, 104553. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery strategies for sustained drug release in the lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, E.Y. Dual functional pullulan-based spray-dried microparticles for controlled pulmonary drug delivery. Int. J. Pharm. 2023, 641, 123057. [Google Scholar] [CrossRef]
- Plaunt, A.J.; Nguyen, T.L.; Corboz, M.R.; Malinin, V.S.; Cipolla, D.C. Strategies to Overcome Biological Barriers Associated with Pulmonary Drug Delivery. Pharmaceutics 2022, 14, 302. [Google Scholar] [CrossRef]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Adichwal, S.A.; Gaikwad, K.R.; Parikh, K.A.; Manvi, F.V. Pulmonary drug delivery: Novel pharmaceutical technologies breathe new life into the lungs. PDA J. Pharm. Sci. Technol. 2011, 65, 513–534. [Google Scholar] [CrossRef]
- Chakravarty, A.; Panchagnula, M.V.; Mohan, A.; Patankar, N.A. Pulmonary drug delivery and retention: A computational study to identify plausible parameters based on a coupled airway-mucus flow model. PLoS Comput. Biol. 2022, 18, e1010143. [Google Scholar] [CrossRef] [PubMed]
- Douafer, H.; Andrieu, V.; Brunel, J.M. Scope and limitations on aerosol drug delivery for the treatment of infectious respiratory diseases. J. Control. Release 2020, 325, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug. Deliv. 2021, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 196, 228–240. [Google Scholar] [CrossRef]
- Dong, W.; Ye, J.; Zhou, J.; Wang, W.; Wang, H.; Zheng, X.; Yang, Y.; Xia, X.; Liu, Y. Comparative study of mucoadhesive and mucus-penetrative nanoparticles based on phospholipid complex to overcome the mucus barrier for inhaled delivery of baicalein. Acta Pharm. Sin. B 2020, 10, 1576–1585. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Harris, R.; Acosta, N.; Heras, A. Chitosan and inhalers: A bioadhesive polymer for pulmonary drug delivery. In Inhaler Devices; Woodhead Publishing: Sawston, UK, 2013; pp. 77–93. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Hidalgo, A.; Garcia-Mouton, C.; Autilio, C.; Carravilla, P.; Orellana, G.; Islam, M.N.; Bhattacharya, J.; Bhattacharya, S.; Cruz, A.; Pérez-Gil, J. Pulmonary surfactant and drug delivery: Vehiculization, release and targeting of surfactant/tacrolimus formulations. J. Control. Release 2021, 329, 205–222. [Google Scholar] [CrossRef]
- Lombry, C.; Edwards, D.A.; Préat, V.; Vanbever, R. Alveolar macrophages are a primary barrier to pulmonary absorption of macromolecules. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1002–L1008. [Google Scholar] [CrossRef]
- Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef]
- Costa, A.; Sarmento, B.; Seabra, V. Targeted Drug Delivery Systems for Lung Macrophages. Curr. Drug Targets 2015, 16, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Misra, A.; Deretic, V. Targeted pulmonary delivery of inducers of host macrophage autophagy as a potential host-directed chemotherapy of tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Choi, Y.; Tanaka, M.; Choi, J. Inhalable nanoparticles delivery targeting alveolar macrophages for the treatment of pulmonary tuberculosis. J. Biosci. Bioeng. 2021, 132, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Pawde, D.M.; Viswanadh, M.K.; Mehata, A.K.; Sonkar, R.; Narendra ; Poddar, S.; Burande, A.S.; Jha, A.; Vajanthri, K.Y.; Mahto, S.K.; et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm. J. 2020, 28, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Saeid, M.E.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Orchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Ribeiro, J.C.V.; Forte, T.C.M.; Tavares, S.J.S.; Andrade, F.K.; Vieira, R.S.; Lima, V. The effects of the molecular weight of chitosan on the tissue inflammatory response. J. Biomed. Mater. Res. A 2021, 109, 2556–2569. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudc, M.; Pavlov, G.; Desbrières, J. Solubilization of Chitosan in Strong Acid Medium. Int. J. Polym. Anal. Charact. 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of acid type for chitosan dissolution on the characteristics and biodegradability of cornstarch/chitosan based films. Int. J. Biol. Macromol. 2019, 138, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Sharmin, N.; Rosnes, J.T.; Prabhu, L.; Böcker, U.; Sivertsvik, M. Effect of Citric Acid Cross Linking on the Mechanical, Rheological and Barrier Properties of Chitosan. Molecules 2022, 27, 5118. [Google Scholar] [CrossRef] [PubMed]
- Tambunan, J.E.; Chamidah, A. Effect of acetic and citric acid solvent combination with cinnamon oil on quality of edible packaging from chitosan. IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012033. [Google Scholar] [CrossRef]
- Sikorski, D.; Gzyra-Jagieła, K.; Draczyński, Z. The Kinetics of Chitosan Degradation in Organic Acid Solutions. Mar Drugs. 2021, 19, 236. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and properties of citric acid cross-linked chitosan/poly(vinyl alcohol) composite films for food packaging applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; Santos, D.M.D.; Fiamingo, A.; Bernardes-Filho, R.; Campana-Filho, S.P.; Azevedo, E.R.; Colnago, L.A. Evaluation of chitosan crystallinity: A high-resolution solid-state NMR spectroscopy approach. Carbohydr. Polym. 2020, 250, 116891. [Google Scholar] [CrossRef]
- González, C.M.; Espinosa, Y.G.; Goycoolea, M. Interaction Between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Aranda-Barradas, M.E.; Trejo-López, S.E.; Del Real, A.; Álvarez-Almazán, S.; Méndez-Albores, A.; García-Tovar, C.G.; González-Díaz, F.R.; Miranda-Castro, S.P. Effect of molecular weight of chitosan on the physicochemical, morphological, and biological properties of polyplex nanoparticles intended for gene delivery. Carbohydr. Polym. Technol. App. 2022, 4, 100228. [Google Scholar] [CrossRef]
- Moura, C.M.; Moura, J.M.; Soares, N.M.; Pinto, L.A.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. Process Intensif. 2011, 50, 351–355. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the Deacetylation Degree of Chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef]
- Foster, L.J.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physicochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef]
- M. Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based. In SystemsFunctional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 457–489. ISBN 978-981-15-0263-7. [Google Scholar]
- Khalaf, E.M.; Abood, N.A.; Atta, R.Z.; Ramírez-Coronel, A.A.; Alazragi, R.; Parra, R.M.R.; Abed, O.H.; Abosaooda, M.; Jalil, A.T.; Mustafa, Y.F.; et al. Bagher Farhood Recent progressions in biomedical and pharmaceutical applications of chitosan nanoparticles: A comprehensive review. International. J. Biol. Macromol. 2023, 231, 123354. [Google Scholar] [CrossRef] [PubMed]
- Limocon, J.R.A.; Madalag, L.M.C.; Reliquias, P.J.B.; Tionko, J.V.S.; Fermin, J.L.; Kee, S.L.; Tan, M.J.T.; Jonco, M.J.J.; Pomperada, M.J.F. Small but Terrible: Utilizing Chitosan-Based Nanoparticles as Drug Carriers to Treat Tuberculosis in the Philippines. Front. Pharmacol. 2021, 12, 752107. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, H.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed]
- Gulati, N.; Dua, K.; Dureja, H. Role of chitosan based nanomedicines in the treatment of chronic respiratory diseases. Int. J. Biol. Macromol. 2021, 185, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Li, Z.Q.; Mo, Z.Q.; Xu, S.; Mao, H.H.; Shi, D.; Li, Z.W.; Dan, X.M.; Luo, X.C. Immunomodulatory Effects of N-Acetyl Chitooligosaccharides on RAW264.7 Macrophages. Mar. Drugs 2020, 18, 421. [Google Scholar] [CrossRef]
- Wu, C.; Dai, Y.; Yuan, G.; Su, J.; Liu, X. Immunomodulatory Effects and Induction of Apoptosis by Different Molecular Weight Chitosan Oligosaccharides in Head Kidney Macrophages From Blunt Snout Bream (Megalobrama amblycephala). Front. Immunol. 2019, 10, 869. [Google Scholar] [CrossRef]
- Oliveira, M.I.; Santos, S.G.; Oliveira, M.J.; Torres, A.L.; Barbosa, M.A. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur. Cells Mater. 2012, 24, 136–153. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Future Sci. OA 2017, 4, FSO225. [Google Scholar] [CrossRef]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Kuen, C.Y.; Masarudin, M.J. Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef] [PubMed]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef]
- Ohya, Y.; Shiratani, M.; Kobayashi, H.; Ouchi, T. Release Behavior of 5-Fluorouracil from Chitosan-Gel Nanospheres Immobilizing 5-Fluorouracil Coated with Polysaccharides and Their Cell Specific Cytotoxicity. J. Macromol. Sci. A 2008, 31, 629–642. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Verma, D.; Okhawilai, M.; Goh, K.L.; Thakur, V.K.; Senthilkumar, N.; Sharma, M.; Uyama, H. Sustainable functionalized chitosan based nano-composites for wound dressings applications: A review. Environ. Res. 2023, 235, 116580. [Google Scholar] [CrossRef]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Borges, O.; Borchard, G.; Verhoef, J.C.; de Sousa, A.; Junginger, H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Pharm. Nanotechnol. 2005, 299, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1998, 63, 125–132. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, M.S.; Yong, C.L.; Sofian, Z.M.; Sahudin, S.; Hasim, N.B.M.; Mahmood, S. Self-assembled chitosan-insulin oral nanoparticles-A critical perspective review. Int. J. Biol. Macromol. 2023, 243, 125125. [Google Scholar] [CrossRef] [PubMed]
- Jalal, R.R.; Ways, T.M.M.; Elella, M.H.A.; Hassan, D.A.; Khutoryanskiy, V.V. Preparation of mucoadhesive methacrylated chitosan nanoparticles for delivery of ciprofloxacin. Int. J. Biol. Macromol. 2023, 242, 124980. [Google Scholar] [CrossRef]
- Marques Gonçalves, M.; Florencio Maluf, D.; Pontarolo, R.; Ketzer Saul, C.; Almouazen, E.; Chevalier, Y. Negatively charged chitosan nanoparticles prepared by ionotropic gelation for encapsulation of positively charged proteins. Int. J. Pharm. 2023, 642, 123164. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Parize, A.L. In vitro cytotoxic and antioxidant evaluation of quercetin loaded in ionic cross-linked chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 74, 103561. [Google Scholar] [CrossRef]
- Kamat, V.; Bodas, D.; Paknikar, K. Chitosan nanoparticles synthesis caught in action using microdroplet reactions. Sci. Rep. 2016, 6, 22260. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Rashid, A.A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Orellano, M.S.; Longo, G.S.; Porporatto, C.; Correa, N.M.; Falcone, R.D. Role of micellar interface in the synthesis of chitosan nanoparticles formulated by reverse micellar method. Colloids Surf. A Physicochem. Eng. 2020, 599, 124876. [Google Scholar] [CrossRef]
- Baldino, L.; Concilio, S.; Cardea, S.; De Marco, I.; Reverchon, E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 2015, 103, 70–76. [Google Scholar] [CrossRef]
- Riegger, B.R.; Bäurer, B.; Mirzayeva, A.; Tovar, G.E.M.; Bach, M. A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohydr. Polym. 2018, 180, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, H.; Li, S.; Wang, Y.; Ma, X.; Tan, M. Ultrasmall Chitosan-Genipin Nanocarriers Fabricated from Reverse Microemulsion Process for Tumor Photothermal Therapy in Mice. Biomacromolecules 2015, 16, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.K.; Wang, S.L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Başaran, E.; Yenilmez, E.; Berkman, M.S.; Büyükköroğlu, G.; Yazan, Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J. Microencapsul. 2014, 31, 49–57. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Zhang, X.; Shi, M.; Li, D.; Wang, Y. Chitosan-Coated Solid Lipid Nano-Encapsulation Improves the Therapeutic Antiairway Inflammation Effect of Berberine against COPD in Cigarette Smoke-Exposed Rats. Can. Respir. J. 2022, 2022, 8509396. [Google Scholar] [CrossRef]
- Da Silva, N.P.; Carmo Rapozo Lavinas Pereira, E.D.; Duarte, L.M.; de Oliveira Freitas, J.C.; de Almeida, C.G.; da Silva, T.P.; Melo, R.C.N.; Morais Apolônio, A.C.; de Oliveira, M.A.L.; de Mello Brandão, H.; et al. Improved anti-Cutibacterium acnes activity of tea tree oil-loaded chitosan-poly(ε-caprolactone) core-shell nanocapsules. Colloids Surf. B Biointerfaces 2020, 196, 111371. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Ali, A.A.; Eid, H.M. Chitosan on the surface of nanoparticles for enhanced drug delivery: A comprehensive review. J. Control. Release 2022, 351, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Changsan, N.; Sinsuebpol, C. Dry powder inhalation formulation of chitosan nanoparticles for co-administration of isoniazid and pyrazinamide. Pharm. Dev. Technol. 2020, 26, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Albetawi, S. Current Research on Spray-Dried Chitosan Nanocomposite Microparticles for Pulmonary Drug Delivery. Pharm. Nanotechnol. 2023, 11, 127–137. [Google Scholar] [CrossRef]
- Scherließ, R.; Bock, S.; Bungert, N.; Neustock, A.; Valentin, L. Particle engineering in dry powders for inhalation. Eur. J. Pharm. Sci. 2022, 172, 106158. [Google Scholar] [CrossRef]
- Wang, D.; Nasab, E.; Athari, S. Study effect of Bacalein encapsulated/loaded chitosan nanoparticle on allergic asthma pathology in mouse model. Saudi J. Biol. Sci. 2021, 28, 4311–4317. [Google Scholar] [CrossRef]
- Ullah, F.; Shah, K.U.; Shah, S.U.; Nawaz, A.; Nawaz, T.; Khan, K.A.; Alserihi, R.F.; Tayeb, H.H.; Tabrez, S.; Alfatama, M. Synthesis, Characterization and In Vitro Evaluation of Chitosan Nanoparticles Physically Admixed with Lactose Microspheres for Pulmonary Delivery of Montelukast. Polymers 2022, 14, 3564. [Google Scholar] [CrossRef]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of Poly(vinyl alcohol) on nanoencapsulation of budesonide in chitosan nanoparticles via ionic gelation and its improved bioavailability. Polymers 2020, 12, 1101. [Google Scholar] [CrossRef]
- Li, Z.; Luo, G.; Hu, W.P.; Hua, J.L.; Geng, S.; Chu, P.K.; Zhang, J.; Wang, H.; Yu, X.F. Mediated drug release from nanovehicles by black phosphorus quantum dots for efficient therapy of chronic obstructive pulmonary disease. Angew. Chem. Int. Ed. Engl. 2020, 59, 20568–20576. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Khallaf, R.A.; Mahmoud, M.O.; Hussein, R.R.S.; El-Kalaawy, A.M.; Abdel-Razik, A.R.H.; Aboud, H.M. Intratracheally inhalable nifedipine-loaded chitosan-PLGA nanocomposites as a promising nanoplatform for lung targeting: Snowballed protection via regulation of TGF-beta/beta-catenin pathway in bleomycin-induced pulmonary fibrosis. Pharmaceuticals 2021, 12, 1225. [Google Scholar] [CrossRef]
- Zhang, G.; Mo, S.; Fang, B.; Zeng, R.; Wang, J.; Tu, M.; Zhao, J. Pulmonary delivery of therapeutic proteins based on zwitterionic chitosan-based nanocarriers for treatment on bleomycin-induced pulmonary fibrosis. Int. J. Biol. Macromol. 2019, 133, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, K.; Efing, J.; Espinosa, Y.G.; Ruland, N.B.; Driessche, W.; Goycoolea, F.M.; Weber, W.M. Capsaicin-Loaded Chitosan Nanocapsules for wtCFTR-mRNA Delivery to a Cystic Fibrosis Cell Line. Biomedicines 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, A.K.; Ruland, N.B.; Goycoolea, F.M.; Weber, W.M. Chitosan nanocomplexes for the delivery of ENaC antisense oligonucleotides to airway epithelial cells. Biomolecules 2020, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Agrawal, A.K.; Anjum, M.M.; Tripathi, M.; Pandey, N.; Bhattacharya, S.; Tilak, R.; Singh, S. DNase-I functionalization of ciprofloxacin-loaded chitosan nanoparticles overcomes the biofilm-mediated resistance of Pseudomonas aeruginosa. Appl. Nanosci. 2019, 10, 563–575. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, W.; Zhu, J.; Zhu, J.; Shen, J.; Liu, Z.; Yang, Y.; Chen, Q. Nanoparticle-Mediated Delivery of Inhaled Immunotherapeutics for Treating Lung Metastasis. Adv. Mater. 2021, 33, 2007557. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Maheshwari, H.; Soniwala, M.; Chavda, J. Pulmonary Delivery of Linezolid Nanoparticles for Treatment of Tuberculosis: Design, Development, and Optimization. J. Pharm. Innov. 2020, 17, 46–59. [Google Scholar] [CrossRef]
- Costabile, G.; Mitidieri, E.; Visaggio, D.; Provenzano, R.; Miró, A.; Quaglia, F.; Angelo, I.; Frangipani, E.; Sorrentino, R.; Visca, P.; et al. Boosting lung accumulation of gallium with inhalable nani-embedded microparticles for the treatment of bacterial pneumonia. Int. J. Pharm. 2022, 629, 122400. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y.; Chen, J.Y.; Chen, J.K. Biphasic release of gentamicin from chitosan/fucoidan nanoparticles for pulmonary delivery. Carbohydr. Polym. 2016, 138, 114–122. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Guo, M.; Liu, C.; Chen, X.; Tao, L.; Zhang, K.; Shen, X. Development of inhalable chitosan-coated oxymatrine liposomes to alleviate RSV-infected mice. Int. J. Mol. Sci. 2022, 23, 15909. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef]
- Tan, C.L.; Chan, Y.; Candasamy, M.; Chellian, J.; Madheswaran, T.; Sakthivel, L.P.; Patel, V.K.; Chakraborty, A.; MacLoughlin, R.; Kumar, D.; et al. Unravelling the molecular mechanisms underlying chronic respiratory diseases for the development of novel therapeutics via in vitro experimental models. Eur. J. Pharmacol. 2022, 919, 174821. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, J.; Wang, C. Self-assembled chitosan nanoparticles for intranasal delivery of recombinant protein interleukin-17 receptor (IL-17RC): Preparation and evaluation in asthma mice. Bioengineered 2021, 12, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Yoon, H.Y.; Kim, H.; Jeon, S.; Hergert, P.; Im, J.; Panyam, J.; Kim, K.; Nho, R.S. The effects of collagen-rich extracellular matrix on the intracellular delivery of glycol chitosan nanoparticles in human lung fibroblasts. Int. J. Nanomed. 2017, 12, 6089–6105. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, K.; Hill, D.; Schoenfisch, M. Antibiofilm and mucolytic action of nitric oxide delivered via gas or macromolecular donor using in vitro and ex vivo models. J. Cyst. Fibros. 2020, 19, 1004–1010. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, Z.; Feng, L.; Deng, L.; Fang, Z.; Liu, Z.; Li, Y.; Wu, X.; Qin, L.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 286, 118237. [Google Scholar] [CrossRef]
- Gonsalves, A.; Sorkhdini, P.; Bazinet, J.; Ghumman, M.; Dhamecha, D.; Zhou, Y.; Menon, J.U. Development and characterization of lung surfactant-coated polymer nanoparticles for pulmonary drug delivery. Biomater. Adv. 2023, 150, 213430. [Google Scholar] [CrossRef]
- Valverde-Fraga, L.; Haddad, R.; Alrabadi, N.; Sánchez, S.; Remuñán-López, C.; Csaba, N. Design and in vitro assessment of chitosan nanocapsules for the pulmonary delivery of rifabutin. Eur. J. Pharm. Sci. 2023, 187, 106484. [Google Scholar] [CrossRef]
- Costa, A.; Pinheiro, M.; Magalhães, J.; Ribeiro, R.; Seabra, V.; Reis, S.; Sarmento, B. The formulation of nanomedicines for treating tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 102–115. [Google Scholar] [CrossRef]
- Hoagland, D.; Liu, J.; Lee, R.B.; Lee, R.E. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 2016, 102, 55–72. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ali, H.; Ahmed, N.; Munir, R.; Talib, S.; Khan, A.S.; Ambrus, R. Drug delivery to macrophages: A review of nanotherapeutics targeted approach for inflammatory disorders and cancer. Expert Opin. Drug Deliv. 2020, 17, 1239–1257. [Google Scholar] [CrossRef]
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, A.; Zeeshan, M.; Burian, K.; Kókai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019. [Google Scholar] [CrossRef]
- Shaji, J.; Shaikh, M. Formulation, optimization, and characterization of biocompatible inhalable d-cycloserine-loaded alginate-chitosan nanoparticles for pulmonary drug delivery. Asian J. Pharm. Clin. Res. 2016, 9, 2455–3891. [Google Scholar] [CrossRef]
- Chogale, M.; Dhoble, S.; Patavale, V. A triple combination ’nano’ dry powder inhaler for tuberculosis: In vitro and in vivo pulmonary characterization. Drug Deliv. Transl. Res. 2021, 11, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Saisivam, S.; Debanth, M.; Omri, A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS ONE 2018, 13, e0190976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Leung, S.S.Y.; Tang, P.; Parumasivam, T.; Loh, Z.H.; Chan, H.K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv. Drug Deliv. Rev. 2015, 8, 83–99. [Google Scholar] [CrossRef]
- Yildiz-Peköz, A.; Akbal, O.; Tekarslan, S.H.; Sagirli, A.O.; Mulazimoglu, L.; Morina, D.; Cevher, E. Preparation and characterization of Doripenem-loaded microparticles for pulmonary delivery. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 347–357. [Google Scholar] [CrossRef]
- Saha, T.; Quiñones-Mateu, M.E.; Das, S.C. Inhaled therapy for COVID-19: Considerations of drugs, formulations and devices. Int. J. Pharm. 2022, 624, 122042. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Deepika; Choudhury, R.; Sonawane, G.A.; Mavinamar, S.; Lyu, X.; Pandey, R.P.; Chang, C.M. Nanoparticles as an effective drug delivery system in COVID-19. Biomed. Pharmacother. 2021, 143, 112162. [Google Scholar] [CrossRef]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef]
- Tan, R.S.L.; Hassandarvish, P.; Chee, C.F.; Chan, L.W.; Wong, T.W. Chitosan and its derivatives as polymeric anti-viral therapeutics and potential anti-SARS-CoV-2 nanomedicine. Carbohydr. Polym. 2022, 290, 119500. [Google Scholar] [CrossRef]
- Tu, B.; Wang, H.; An, X.; Qu, J.; Li, Q.; Gao, Y.; Shi, M.; Qiu, H.; Huang, Y. Inhaled heparin polysaccharide nanodecoy against SARS-CoV-2 and variants. Acta Pharm. Sin. B 2022, 12, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin Encapsulated Nanoparticles as a Pulmonary Delivery System. Oxid. Med. Cell. Longev. 2022, 2022, 1422929. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Liu, Y.; Tang, Y.; Chen, J.; Li, S.; Pu, J.; Han, L. GABAB receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2018, 179, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Rosière, R.; Woensel, M.V.; Gelbcke, M.; Mathieu, V.; Hecq, J.; Mathivet, T.; Vermeersch, M.; Antuérpia, P.V.; Amighi, K.; Wauthoz, N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol. Pharm. 2018, 15, 899–910. [Google Scholar] [CrossRef]
- Petkar, K.C.; Chavhan, S.; Kunda, N.; Saleem, E.; Somavarapu, S.; Taylor, K.M.; Sawant, K.K. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: Optimization by factorial design. AAPS PharmSciTech 2018, 19, 1758–1772. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165, 445–459. [Google Scholar] [CrossRef]
- Ainali, N.M.; Xanthopoulou, E.; Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Microencapsulation of fluticasone propionate and salmeterol xinafoate in modified chitosan microparticles for release optimization. Molecules 2020, 25, 3888. [Google Scholar] [CrossRef]
- Nel, A.E.; Meng, H.; Allen, S. Gsk3 Inhibitor-Loaded Nano Formulations as a Cancer Immunotherapeutic. Patent WO2022006083A1, 1 January 2022. [Google Scholar]
- Hubbard, B.; Serrano-Wu, M. Method of Use for Apoe Peptides. Patent WO2023288316A1, 19 January 2023. [Google Scholar]
- Yantasee, W.; Ngamcherdtrakul, W.; Lund, A.; Reda, M. Immunotherapeutic Constructs and methods of Their Use. Patent WO2021011496A1, 21 January 2021. [Google Scholar]
- Ramirez, C.; Hauser, A.; Bar-Sagi, D.; Koide, A.; Koide, S. Npc1 Monobodies and Monobody Conjugates Thereof. Patent WO2022103840A2, 19 May 2022. [Google Scholar]
- Takeuchi, H.; Nakano, K.; Toyobuku, H. Transpulmonary Liposome for Controlling Drug Arrival. Patent AU2014204483A1, 7 August 2014. [Google Scholar]
- Zhang, W.; Liu, K.; Tang, J.; Zheng, Z. Quercetin and Paclitaxel Co-Transportation Pulmonary Inhaled Nanometer Targeted Porous Polymer Particle and Preparation Method Thereof. Patent CN106309411A, 11 January 2017. [Google Scholar]
- Zhang, W.; Liu, K.; Tang, J.; Zheng, Z. A Pulmonary Inhaled Chitosan-Based Nano Targeting Polymer Particles and Its Production Method Thereof. Patent CN106265607A, 4 January 2017. [Google Scholar]
- Ak, G. Nano-Delivery System for Inhaled Chemotherapy. Patent WO2022119528A1, 9 June 2022. [Google Scholar]
- Vesco, D. Therapeutic Methods and Compositions Comprising Magnetizable Nanoparticles. Patent WO2022187556A1, 9 September 2022. [Google Scholar]
- Kheir, J.; Polizzotti, B.D. Hollow Particles Encapsulating a Biological Gas and Methods of Use. Patent WO2014143808A1, 18 September 2014. [Google Scholar]
- Crook, Z.; Olson, J.; Nairn, N.W.; Correnti, C. PD-L1 Binding Peptides and Peptide Complexes and Methods of Use Thereof. Patent WO2022115719A1, 2 June 2022. [Google Scholar]
- On Drug Delivery. Available online: https://ondrugdelivery.com/chitosan-nanoparticles-suitable-for-aerosol-treatment-of-covid-19-patients/ (accessed on 5 September 2023).
- Chaudhary, T. Chitosan Market Research Report Information by Source (Shrimps, Prawns, Crabs, Lobsters, Fungi, and Others), by Application (Food & Beverages, Pharmaceuticals & Nutraceuticals, Cosmetics & Personal care, Agriculture, and Others), and by Region (North America, Europe, Asia-Pacific, and Rest of the World)–Market Forecast Till 2030. Market Research Future. Available online: https://www.marketresearchfuture.com/reports/chitosanmarket-2269 (accessed on 5 September 2023).









| Disease | Drug | Limitations | Carrier | Main Results | Ref. |
|---|---|---|---|---|---|
| Asthma | Ferulic Acid | Low bioavailability and short half-life | Hyaluronic acid-coated CS NP | Improved drug interaction and transport across the mucus layer; increased therapeutic efficacy | [18] |
| Budesonide | Low bioavailability | CS-coated PLGA NP | Improved bioavailability and in vivo lung deposition in animal model | [19] | |
| Baicalein | Low bioavailability | CS NP | Nanoparticles control the immune-allergy-inflammatory response of asthma in mice | [119] | |
| Montelukast | Significant hepatic metabolism after oral administration | CS NP | DPI formulation showed Optimum deposition in the deep lung | [120] | |
| COPD | Budesonide | Low aqueous solubility and bioavailability | CS NP | Enhancement of drug solubility | [121] |
| Amikacin | Poor lung penetration after endovenous administration | PEG-CS NP combined with black phosphorus quantum dots | Improved mucus penetration and antibacterial activity | [122] | |
| Pulmonary fibrosis | Nifedipine | Low bioavailability | CS-PLGA NP | Reduced markers of pulmonary fibrosis and oxidative stress | [123] |
| IPF | msFGFR2c | Low bioavailability | Phosphoryl- choline-CS NP | Enhanced antifibrotic efficacy, reduced inflammatory cytokines, decreased pulmonary fibrosis score and collagen deposition | [124] |
| CF | Ciprofloxacin | Microbial resistance | ALG-lyase-functionalized CS NP | Higher inhibitory effect on P. aeruginosa biofilm | [20] |
| wtCFTR-mRNA | Low stability; low transfection efficiency | CS-lecithin oil-core nanocapsules | Restored CFTR function in the cystic fibrosis cell line | [125] | |
| Antisense oligonucleotide (ASO) | Low stability | CS/ASO nanocomplex | Significant downregulation of ENaC activity in human respiratory epithelial cells | [126] | |
| Tobramycin | High frequency of administration; ototoxic and nephrotoxic effects; bacterial resistance | SLPICS-functionalized ALG/CS NP | Inhibition of P. aeruginosa in vitro; reduction in inflammatory response; improvement in interaction with CF mucus | [22] | |
| Ciprofloxacin | Microbial resistance | DNase-I-functionalized CS NP | Prolonged microbial inhibition, prevention of biofilm formation and biofilm dispersal potential | [127] | |
| Lung cancer | Resveratrol | Low solubility | CS/lecithin nanocomplex | Enhanced antitumor activity; increased selectivity in A549 cells | [22] |
| aPD-L1 | Low stability; unwanted adverse effects | CS/aPD-L1 nanocomplex | Improved lung adhesion and permeation; enhanced therapeutic efficacy | [128] | |
| Tuberculosis | Bedaquiline | Prolonged treatment; unwanted adverse effects | CS NP | Reduction in toxic effects; Increased drug concentration in the lungs | [15] |
| Linezolid | Unwanted adverse effects | CS NPs | Improved deep lung deposition in vitro | [129] | |
| Pneumonia | Gallium [Ga(III)] | Nephrotoxicity | Hyaluronic acid-CS NP | Improvement in Ga(III) persistence in the lungs and preventing its accumulation in the kidney | [130] |
| Gentamicin | Low bioavailability; unwanted adverse effects | CS/Fucoidan NP | Improved antibacterial activity; reduced systemic toxicity | [131] | |
| RSV | Oxymatrine | Enzymatic degradation; poor lung penetration | CS-coated liposomes | Enhanced distribution and retention of oxymatrine in lung tissue in vivo | [132] |
| COVID-19 | Silymarin and curcumin | Low penetration and adsorption in the lungs | CS-coated BSA NP | Reduced inflammation; enhanced antiviral activity in vitro | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacaron, T.M.; Silva, M.L.S.e.; Costa, M.P.; Silva, D.M.e.; Silva, A.C.; Apolônio, A.C.M.; Fabri, R.L.; Pittella, F.; Rocha, H.V.A.; Tavares, G.D. Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery. Polymers 2023, 15, 3849. https://doi.org/10.3390/polym15183849
Zacaron TM, Silva MLSe, Costa MP, Silva DMe, Silva AC, Apolônio ACM, Fabri RL, Pittella F, Rocha HVA, Tavares GD. Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery. Polymers. 2023; 15(18):3849. https://doi.org/10.3390/polym15183849
Chicago/Turabian StyleZacaron, Thiago Medeiros, Mariana Leite Simões e Silva, Mirsiane Pascoal Costa, Dominique Mesquita e Silva, Allana Carvalho Silva, Ana Carolina Morais Apolônio, Rodrigo Luiz Fabri, Frederico Pittella, Helvécio Vinícius Antunes Rocha, and Guilherme Diniz Tavares. 2023. "Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery" Polymers 15, no. 18: 3849. https://doi.org/10.3390/polym15183849
APA StyleZacaron, T. M., Silva, M. L. S. e., Costa, M. P., Silva, D. M. e., Silva, A. C., Apolônio, A. C. M., Fabri, R. L., Pittella, F., Rocha, H. V. A., & Tavares, G. D. (2023). Advancements in Chitosan-Based Nanoparticles for Pulmonary Drug Delivery. Polymers, 15(18), 3849. https://doi.org/10.3390/polym15183849