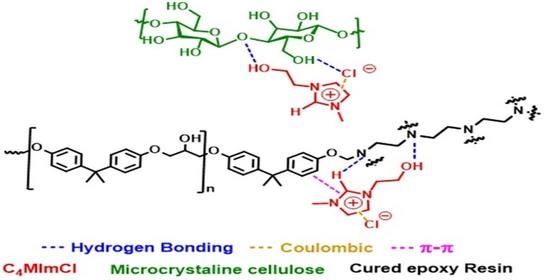
Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. MCC Treatment and Characterization
2.2. Epoxy Composites Preparation and Characterization
3. Results and Discussion
3.1. MCC Surface Treatment
3.2. Interaction of IL with Epoxy Matrix
3.3. MCC-IL/Epoxy Composites’ Properties
3.3.1. Curing Kinetic
3.3.2. Tensile, Flexural and Fracture Toughness Behavior
3.3.3. Viscoelastic Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epoxy Resin Market Size, Share, Overview—Industry Report, 2020–2026. Available online: https://www.polarismarketresearch.com/industry-analysis/epoxy-resins-market (accessed on 24 September 2021).
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and Application of Epoxy Resins: A Review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- McCoy, J.D.; Ancipink, W.B.; Clarkson, C.M.; Kropka, J.M.; Celina, M.C.; Giron, N.H.; Hailesilassie, L.; Fredj, N. Cure Mechanisms of Diglycidyl Ether of Bisphenol A (DGEBA) Epoxy with Diethanolamine. Polymer 2016, 105, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Li, J.; Qi, Y.; Zhang, S.; Wang, J.; Wei, Z.; Pang, H.; Jian, X.; Weng, Z. Engineering Double Load-Sharing Network in Thermosetting: Much More than Just Toughening. Macromolecules 2022, 55, 9502–9512. [Google Scholar] [CrossRef]
- Leguizamon, S.C.; Powers, J.; Ahn, J.; Dickens, S.; Lee, S.; Jones, B.H. Polymerization-Induced Phase Separation in Rubber-Toughened Amine-Cured Epoxy Resins: Tuning Morphology from the Nano- To Macro-Scale. Macromolecules 2021, 54, 7796–7807. [Google Scholar] [CrossRef]
- Dittanet, P.; Pearson, R.A. Effect of Silica Nanoparticle Size on Toughening Mechanisms of Filled Epoxy. Polymer 2012, 53, 1890–1905. [Google Scholar] [CrossRef]
- Norman, D.A.; Robertson, R.E. Rigid-Particle Toughening of Glassy Polymers. Polymer 2003, 44, 2351–2362. [Google Scholar] [CrossRef]
- Kerche, E.F.; da Silva, V.D.; Fonseca, E.; Salles, N.A.; Schrekker, H.S.; Amico, S.C. Epoxy-Based Composites Reinforced with Imidazolium Ionic Liquid-Treated Aramid Pulp. Polymer 2021, 226, 123787. [Google Scholar] [CrossRef]
- Hameed, A.; Islam, M.; Ahmad, I.; Mahmood, N.; Saeed, S.; Javed, H. Thermal and Mechanical Properties of Carbon Nanotube/Epoxy Nanocomposites Reinforced with Pristine and Functionalized Multiwalled Carbon Nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar] [CrossRef]
- Mostovoy, A.; Shcherbakov, A.; Yakovlev, A.; Arzamastsev, S.; Lopukhova, M. Reinforced Epoxy Composites Modified with Functionalized Graphene Oxide. Polymers 2022, 14, 338. [Google Scholar] [CrossRef]
- Sun, J.; Bai, J.; Li, J. The Synergistic Toughening and Strengthening Effects of Cork Particles and Nanocellulose on Rosin-Based Epoxy Resin. Polymers 2022, 14, 5064. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; da Silva, H.S.P.; Zattera, A.J.; Amico, S.C. Dynamic Mechanical Properties of Curaua Composites. J. Appl. Polym. Sci. 2012, 125, E110–E116. [Google Scholar] [CrossRef]
- Lavoratti, A.; Scienza, L.C.; Zattera, A.J. Dynamic-Mechanical and Thermomechanical Properties of Cellulose Nanofiber/Polyester Resin Composites. Carbohydr. Polym. 2015, 136, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kerche, E.F.; Bock, D.N.; de Avila Delucis, R.; Magalhães, W.L.E.; Amico, S.C. Micro Fibrillated Cellulose Reinforced Bio-Based Rigid High-Density Polyurethane Foams. Cellulose 2021, 28, 4313–4326. [Google Scholar] [CrossRef]
- Neves, R.M.; Zattera, A.J.; Campos Amico, S. Enhancing Thermal and Dynamic-mechanical Properties of Epoxy Reinforced by Amino-functionalized Microcrystalline Cellulose. J. Appl. Polym. Sci. 2021, 138, 51329. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. Recent Studies on Modified Cellulose/Nanocellulose Epoxy Composites: A Systematic Review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. The Influence of Silane Surface Modification on Microcrystalline Cellulose Characteristics. Carbohydr. Polym. 2020, 230, 115595. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, J.; Li, X.; Wen, Y.; Li, X.; Shi, D.; Xue, Z. Epoxy/Ionic Liquid-like MWCNTs Composites with Improved Processability and Mechanical Properties. Compos. Commun. 2019, 15, 46–52. [Google Scholar] [CrossRef]
- Neves, R.M.; Kerche, E.F.; Monticeli, F.M.; Heitor Luiz, O.J. Modeling of the Creep Behavior of Epoxy/Yerba-Mate Residue Composites. J. Compos. Mater. 2022, 56, 2755–2764. [Google Scholar] [CrossRef]
- Kerche, E.F.; Fonseca, E.; Schrekker, H.S.; Amico, S.C. Ionic Liquid-Functionalized Reinforcements in Epoxy-Based Composites: A Systematic Review. Polym. Compos. 2022, 43, 5783–5801. [Google Scholar] [CrossRef]
- Luiz, H.; Roberta, O.; Neves, M.; Fischer Kerche, E.; Maciel, F.; Ademir, M.; Zattera, J.; Ornaghi, H.L.; Neves, R.M.; Kerche, E.F.; et al. A Simple Model to Fit and Interpret Creep Curves-Behaviour of Modified Micro-Cellulose Particulate Composites. Cellulose 2022, 29, 8283–8292. [Google Scholar] [CrossRef]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing Effects of Aminosilane-Functionalized Graphene on the Tribological and Mechanical Behaviors of Epoxy Nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Yue, L.; Maiorana, A.; Khelifa, F.; Patel, A.; Raquez, J.M.; Bonnaud, L.; Gross, R.; Dubois, P.; Manas-Zloczower, I. Surface-Modified Cellulose Nanocrystals for Biobased Epoxy Nanocomposites. Polymer 2018, 134, 155–162. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Qi, A.; Raymonde, N.; Naguib, H.E. Ionic Liquids Facilitated Dispersion of Chitin Nanowhiskers for Reinforced Epoxy Composites. Carbohydr. Polym. 2020, 247, 116746. [Google Scholar] [CrossRef]
- da Silva, V.D.; Jacobi, M.M.; Schrekker, H.S.; Amico, S.C. Aramid Pulp with Physisorbed Imidazolium Ionic Liquids for Solvent-Casted Enhanced Styrene-Butadiene Rubber Composites. J. Appl. Polym. Sci. 2018, 135, 46693. [Google Scholar] [CrossRef]
- da Silva, V.D.; Jacobi, M.M.; Schrekker, H.S.; Amico, S.C. Imidazolium Ionic Liquid Compatibilizers in Melt—Blended Styrene—Butadiene Rubber/Aramid Pulp Composites. Polym. Bull. 2019, 76, 3451–3462. [Google Scholar] [CrossRef]
- Moraes, C.V.; da Silva, V.D.; Castegnaro, M.V.; Morais, J.; Schrekker, H.S.; Amico, S.C. Lightweight Composites through Imidazolium Ionic Liquid Enhanced Aramid—Epoxy Resin Interactions. ACS Appl. Polym. Mater. 2020, 2, 1754–1763. [Google Scholar] [CrossRef]
- Kerche, E.F.; da Silva, V.D.; da Silveira Jankee, G.; Schrekker, H.S.; de Avila Delucis, R.; Irulappasamy, S.; Amico, S.C. Aramid Pulp Treated with Imidazolium Ionic Liquids as a Filler in Rigid Polyurethane Bio-Foams. J. Appl. Polym. Sci. 2021, 138, 50492. [Google Scholar] [CrossRef]
- Kerche, E.F.; Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Schrekker, H.S. The Influence of Ionic Liquid Concentration on Microcrystalline Cellulose Modification. Carbohydr. Polym. Technol. Appl. 2022, 3, 100211. [Google Scholar] [CrossRef]
- Santos, D.F.; Carvalho, A.P.A.; Soares, B.G. Phosphonium-Based Ionic Liquid as Crosslinker/Dispersing Agent for Epoxy/Carbon Nanotube Nanocomposites: Electrical and Dynamic Mechanical Properties. J. Mater. Sci. 2020, 55, 2077–2089. [Google Scholar] [CrossRef]
- Gholami, H.; Arab, H.; Mokhtarifar, M.; Maghrebi, M.; Baniadam, M. The Effect of Choline-Based Ionic Liquid on CNTs’ Arrangement in Epoxy Resin Matrix. Mater. Des. 2016, 91, 180–185. [Google Scholar] [CrossRef]
- Gisbert, P.; Trillo, P.; Pastor, I.M. Comparative Study of Catalytic Systems Formed by Palladium and Acyl-Substituted Imidazolium Salts. ChemistrySelect 2018, 3, 887–893. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Yu, S.; Liu, S.; Liu, F.; Xie, C. Stability and Activity of Cellulase Modified with Polyethylene Glycol (PEG) at Different Amino Groups in the Ionic Liquid [C2OHmim][OAc]. Chem. Eng. Commun. 2018, 205, 986–990. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Y.; Zhang, N.; Zhang, L.; Darensbourg, D.J. One-Pot Synthesis of Ion-Containing CO2-Based Polycarbonates Using Protic Ionic Liquids as Chain Transfer Agents. Macromolecules 2018, 51, 9122–9130. [Google Scholar] [CrossRef]
- Prabhakara, M.D.; Maiti, B. Ionic Liquid-Immobilized Proline(s) Organocatalyst-Catalyzed One-Pot Multi-Component Mannich Reaction under Solvent-Free Condition. Res. Chem. Intermed. 2020, 46, 2381–2401. [Google Scholar] [CrossRef]
- da Silva, A.A.X.; Souza, J.A.; Manes, A.; Amico, S.C. In-Plane Permeability and Mechanical Properties of R-Glass/Aramid Hybrid Composites. J. Mater. Eng. Perform. 2020, 29, 4484–4492. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Bolner, A.S.; Fiorio, R.; Zattera, A.J.; Amico, S.C. Mechanical and Dynamic Mechanical Analysis of Hybrid Composites Molded by Resin Transfer Molding. J. Appl. Polym. Sci. 2010, 118, 887–896. [Google Scholar] [CrossRef]
- Neves, R.M.; Lopes, K.S.; Zimmermann, M.G.V.; Poletto, M.; Zattera, A.J. Cellulose Nanowhiskers Extracted from Tempo- Oxidized Curaua Fibers Cellulose Nanowhiskers Extracted from Tempo-Oxidized Curaua. J. Nat. Fibers 2020, 17, 1355–1365. [Google Scholar] [CrossRef]
- da Silva, V.D.; de Barros, Í.R.; da Conceição, D.K.S.; de Almeida, K.N.; Schrekker, H.S.; Amico, S.C.; Jacobi, M.M. Aramid Pulp Reinforced Hydrogenated Nitrile Butadiene Rubber Composites with Ionic Liquid Compatibilizers. J. Appl. Polym. Sci. 2020, 137, 48702. [Google Scholar] [CrossRef]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-Free Acetylation of Cellulose Nanofibers for Improving Compatibility and Dispersion. Carbohydr. Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zarycz, M.N.C.; Fonseca Guerra, C. NMR 1H-Shielding Constants of Hydrogen-Bond Donor Reflect Manifestation of the Pauli Principle. J. Phys. Chem. Lett. 2018, 9, 3720–3724. [Google Scholar] [CrossRef]
- Neves, R.M.; Vanzetto, A.B.; Lazzari, L.K.; Zattera, A.J. Thermal and Dynamic Mechanical Behavior of Epoxy Composites Reinforced with Post-consumed Yerba Mate. J. Appl. Polym. Sci. 2021, 138, 50438. [Google Scholar] [CrossRef]
- Lavoratti, A.; Zattera, A.J.; Amico, S.C. Mechanical and Dynamic-Mechanical Properties of Silane-Treated Graphite Nanoplatelet/Epoxy Composites. J. Appl. Polym. Sci. 2018, 135, 46724. [Google Scholar] [CrossRef]
- Jung, J.; Sodano, H.A. High Strength Epoxy Nanocomposites Reinforced by Epoxy Functionalized Aramid Nanofibers. Polymer 2020, 195, 122438. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Effects of Ionic Liquid on Cellulosic Nanofiller Filled Natural Rubber Bionanocomposites. J. Colloid Interface Sci. 2021, 591, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Influence of Ionic Liquid on Rheological Behaviors of Candle Soot and Cellulose Nanocrystal Filled Natural Rubber Nanocomposites. Compos. Commun. 2022, 33, 101214. [Google Scholar] [CrossRef]
- Okoli, O.I.; Smith, G.F. The Effect of Strain Rate and Fibre Content on the Poisson’s Ratio of Glass/Epoxy Composites. Compos. Struct. 2000, 48, 157–161. [Google Scholar] [CrossRef]
- Neves, R.M.; Kerche, E.F.; Zattera, A.J.; Amico, S.C. Hybridization Effect of Functionalized Microcrystalline Cellulose and Liquid Acrylonitrile Butadiene Rubber on Epoxy. J. Compos. Mater. 2022, 56, 2867–2877. [Google Scholar] [CrossRef]
- Kang, W.S.; Rhee, K.Y.; Park, S.J. Influence of Surface Energetics of Graphene Oxide on Fracture Toughness of Epoxy Nanocomposites. Compos. B Eng. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Tastanova, L.; Kadykova, Y.; Kalganova, S.; Lopukhova, M. Reinforcement of Epoxy Composites with Application of Finely-Ground Ochre and Electrophysical Method of the Composition Modification. Polymers 2020, 12, 1437. [Google Scholar] [CrossRef]
- Fonseca, E.; Demétrio da Silva, V.; Klitzke, J.S.; Schrekker, H.S.; Amico, S.C. Imidazolium Ionic Liquids as Fracture Toughening Agents in DGEBA-TETA Epoxy Resin. Polym. Test 2020, 87, 106556. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y. Large Amplitude Oscillatory Rheology of Silica and Cellulose Nanocrystals Filled Natural Rubber Compounds. J. Colloid Interface Sci. 2021, 588, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, H.L.; Neves, R.M.; Monticeli, F.M.; Almeida, J.H.S. Viscoelastic Characteristics of Carbon Fiber-Reinforced Epoxy Filament Wound Laminates. Compos. Commun. 2020, 21, 100418. [Google Scholar] [CrossRef]
- Júnior, J.H.S.A.; Júnior, H.L.O.; Amico, S.C.; Amado, F.D.R. Study of Hybrid Intralaminate Curaua/Glass Composites. Mater. Des. 2012, 42, 111–117. [Google Scholar] [CrossRef]
- Pothan, L.A.; Oommen, Z.; Thomas, S. Dynamic Mechanical Analysis of Banana Fiber Reinforced Polyester Composites. Compos. Sci. Technol. 2003, 63, 283–293. [Google Scholar] [CrossRef]










| Sample | Tonset (°C) | Tmax (°C) | Weight Loss at Tmax (%) | Weight Loss at 374 °C (%) |
|---|---|---|---|---|
| MCC MCC_C4 MCC_C2OH MCC_HO2 MCC_(HO2)2 | 322.9 302.6 301.7 329.5 332.6 | 352.3 377.3 367.8 365.1 366.0 | 61.5 68.5 65.9 62.5 61.3 | 93.1 62.1 77.5 72.3 78.4 |
| Sample | Tonset (°C) | Tpeak (°C) | Heat of Reaction (J/g) |
|---|---|---|---|
| E E/MCC10 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 62.0 61.6 61.2 61.4 61.1 61.3 | 110.6 107.8 111.3 106.7 111.7 111.0 | 427.5 459.3 418.9 410.4 413.7 397.2 |
| Sample | Tensile Modulus (MPa) 1 | Tensile Strength (MPa) 1 | Poisson’s Ratio (ν) 1 | Shear Modulus (MPa) 1 |
|---|---|---|---|---|
| E E/MCC5 E/MCC10 E/MCC_C45 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 2696 3.5 AB 2894 11.3 BC 2487 10.8 A 2608 6.1 AB 3139 7.4 CD 3285 6.8 DE 2758 9.3 AB 3535 10.9 E | 27.4 8.7 ABC 44.5 6.4 G 43.5 5.9 FG 33.8 9.9 CD 28.8 11.0 B 37.6 13.5 DE 24.5 9.9 A 39.8 12.7 EF | 0.26 11.9 D 0.21 9.8 C 0.16 1.2 B 0.18 2.3 BC 0.21 4.4 BC 0.19 2.4 BC 0.14 3.6 B 0.12 2.2 A | 1827.4 9.3 BC 1952.6 11.9 C 1498.0 11.0 A 1615.2 5.3 AB 1879.4 14.9 BC 2018.9 11.7 CD 1999.5 14.4 CD 2274.7 13.6 D |
| Sample | Flexural Modulus (MPa) 1 | Flexural Strength (MPa) 1 | Strain at Flexural Break (%) 1 |
|---|---|---|---|
| E E/MCC5 E/MCC10 E/MCC_C45 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 2287 11.9 A 2313 13.5 A 2302 4.7 A 2593 12.9 AB 2723 6.7 B 2734 13.7 B 2502 13.2 AB 2671 13.3 B | 65.6 10.7 BC 73.8 11.2 CD 50.9 9.7 A 84.6 12.9 E 76.3 8.6 DE 63.1 13.8 B 64.6 5.1 B 67.3 12.8 BC | 11.6 5.6 D 3.7 13.5 BC 2.7 13.2 A 4.3 13.0 C 3.4 8.3 B 2.5 13.7 A 2.7 12.0 A 2.5 14.9 A |
| Sample | KIC (MPa.m−2) 1 | GIC (kJ.m−2) 1 |
|---|---|---|
| E E/MCC5 E/MCC10 E/MCC_C45 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 1.57 12.0 B 1.32 13.4 A 1.40 5.6 AB 1.43 17.6 AB 1.39 11.9 AB 1.37 9.38 AB 1.30 13.6 A 1.30 13.1 A | 8.16 10.4 AB 8.95 4.7 AB 8.57 7.7 AB 8.92 10.2 AB 10.03 13.2 C 9.23 11.7 BC 7.99 8.6 A 9.05 4.3 B |
| Sample | Ec/Em (at 40 °C) | Ec/Em (at 140 °C) | C (40/140 °C) |
|---|---|---|---|
| E/MCC5 E/MCC10 E/MCC_C45 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 1.16 1.31 2.55 1.12 1.15 2.05 2.07 | 1.59 1.74 3.69 1.60 1.67 2.65 2.41 | 0.73 0.75 0.69 0.70 0.68 0.78 0.86 |
| Sample | Tg (°C) 1 | Peak Height 1 | FWHM (°C) 1 |
|---|---|---|---|
| E E/MCC5 E/MCC10 E/MCC_C45 E/MCC_C410 E/MCC_HO210 E/MCC_(HO2)210 E/MCC_C2OH10 | 101.88 101.63 102.42 106.51 101.86 101.20 100.88 101.38 | 0.76 0.69 0.69 0.61 0.76 0.70 0.76 0.78 | 17.6 18.3 19.33 17.48 17.31 18.28 18.67 17.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerche, E.F.; Kairytė, A.; Członka, S.; da Silva, V.D.; Salles, N.A.; Schrekker, H.S.; Amico, S.C. Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites. Polymers 2023, 15, 333. https://doi.org/10.3390/polym15020333
Kerche EF, Kairytė A, Członka S, da Silva VD, Salles NA, Schrekker HS, Amico SC. Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites. Polymers. 2023; 15(2):333. https://doi.org/10.3390/polym15020333
Chicago/Turabian StyleKerche, Eduardo Fischer, Agnė Kairytė, Sylwia Członka, Vinícius Demétrio da Silva, Nicholas Alves Salles, Henri Stephan Schrekker, and Sandro Campos Amico. 2023. "Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites" Polymers 15, no. 2: 333. https://doi.org/10.3390/polym15020333
APA StyleKerche, E. F., Kairytė, A., Członka, S., da Silva, V. D., Salles, N. A., Schrekker, H. S., & Amico, S. C. (2023). Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites. Polymers, 15(2), 333. https://doi.org/10.3390/polym15020333