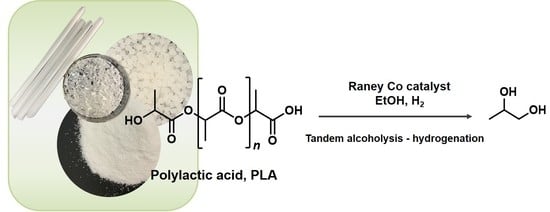
One-Pot Tandem Alcoholysis-Hydrogenation of Polylactic Acid to 1,2-Propanediol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Typical Alcoholysis and Hydrogenation Process
2.3. Analysis
2.4. Digestion PLA in Ethanol
2.5. Calcination of PLA Straws
2.6. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
2.7. Box-Benhken Optimization Design
3. Results and Discussion
3.1. Analysis of Alcoholysis and Hydrogenation Products
3.2. Effect of Single Factor
3.3. Optimization of Reaction Procedure
0.2 BC − 4.3 A2 − 18.0 B2 − 4.0 C2
3.4. Verification for Optimal Model
3.5. Reusability Test of Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Maga, D.; Hiebel, M.; Thonemann, N. Life cycle assessment of recycling options for polylactic acid. Resour. Conserv. Recy. 2019, 149, 86–96. [Google Scholar] [CrossRef]
- Lorenzo, M.L.D.; Androsch, R. Applications of Poly(Lactic Acid) in Commodities and Specialties, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–16. [Google Scholar]
- Bioplastics Market Development Update 2019. Available online: https://docs.european-bioplastics.org/conference/Report_Bioplastics_Market_Data_2020_short_version.pdf (accessed on 22 February 2021).
- Hong, M.; Chen, Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Rossi, V.; Cleeve-Edwards, N.; Lundquist, L.; Schenker, U.; Dubois, C.; Humbert, S.; Jolliet, O. Life cycle assessment of end-of-life options for two biodegradable packaging materials: Sound application of the European waste hierarchy. J. Clean. Prod. 2015, 86, 132–145. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolic, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wei, S.; Tan, H.; Huang, Y.; Zhang, Y. Progress in upcycling polylactic acid waste as an alternative carbon source: A review. Chem. Eng. J. 2022, 446, 136881. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Kolokotsiou, L.; Vouvoudi, E.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Depolymerization of PLA by phase transfer catalysed alkaline hydrolysis in a microwave reactor. J. Polym. Environ. 2020, 28, 1664–1672. [Google Scholar] [CrossRef]
- Cristina, A.M.; Sara, F.; Fausto, G.; Vincenzo, P.; Rocchina, S.; Claudio, V. Degradation of post-consumer PLA: Hydrolysis of polymeric matrix and oligomers stabilization in aqueous phase. J. Polym. Environ. 2018, 26, 4396–4404. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers. Manage. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Garg, M.; White, S.R.; Sottos, N.R. Rapid degradation of poly(lactic acid) with organometallic catalysts. ACS Appl. Mater. Interface 2019, 11, 46226–46232. [Google Scholar] [CrossRef]
- McKeown, P.; Kamran, M.; Davidson, M.G.; Jones, M.D.; Román-Ramírez, L.A.; Wood, J. Organocatalysis for versatile polymer degradation. Green Chem. 2020, 22, 3721–3726. [Google Scholar] [CrossRef]
- Majgaonkar, P.; Hanich, R.; Malz, F.; Brüll, R. Chemical recycling of post-consumer pla waste for sustainable production of ethyl lactate. Chem. Eng. J. 2021, 423, 129952. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, K.; Fu, J.; Tan, Z.; Qin, L.; Duan, P.; Xu, Y.; Kang, S. Near-zero-waste hydrogenolysis of poly(lactic acid) to biofuel. Fuel 2023, 334, 126609. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manage. 2021, 127, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiao, Y.; Gao, Z.; Xu, Y.; Fu, L.; Fu, H.; Zhou, W.; Hu, C.; Liu, G.; Wang, M.; et al. Catalytic amination of polylactic acid to alanine. J. Am. Chem. Soc. 2021, 143, 16358–16363. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Dubrovina, N.V.; Schulze, J.; Tietz, W.; Kuhlein, K.; Borner, A. Propane-1,2-diols from dilactides, oligolactides, or poly-L-lactic acid (PLLA): From plastic waste to chiral bulk chemicals. Chem. Eur. J. 2014, 20, 957–960. [Google Scholar] [CrossRef]
- Krall, E.M.; Klein, T.W.; Andersen, R.J.; Nett, A.J.; Glasgow, R.W.; Reader, D.S.; Dauphinais, B.C.; Mc Ilrath, S.P.; Fischer, A.A.; Carney, M.J.; et al. Controlled hydrogenative depolymerization of polyesters and polycarbonates catalyzed by ruthenium(ii) PNN pincer complexes. Chem. Commun. 2014, 50, 4884–4887. [Google Scholar] [CrossRef]
- Vivek, N.; Hazeena, S.H.; Alphy, M.P.; Kumar, V.; Magdouli, S.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in microbial biosynthesis of C3 - C5 diols: Genetics and process engineering approaches. Bioresour. Technol. 2021, 322, 124527. [Google Scholar] [CrossRef]
- Kirk, R.E. Encyclopedia of Chemical Technology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 157–170. [Google Scholar]
- Tabassum, N.; Pothu, R.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Mameda, N.; et al. Heterogeneous catalysts for conversion of biodiesel-waste glycerol into high-added-value chemicals. Catalysts 2022, 12, 767. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Xiao, Z.; Jin, S.; Sha, G.; Williams, C.T.; Liang, C. Two-step conversion of biomass-derived glucose with high concentration over Cu–Cr catalysts. Ind. Eng. Chem. Res. 2014, 53, 8735–8743. [Google Scholar] [CrossRef]
- Dastidar, R.G.; Galebach, P.H.; Lanci, M.P.; Wang, C.; Du, Y.; Huber, G.W. Elucidation of reaction network and kinetics between cellulose-derived 1,2-propanediol and methanol for one-pot biofuel production. Green Chem. 2022, 24, 350–364. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X.; Pidko, E.A.; Hensen, E.J.M. Hydrogenation of lactic acid to 1,2-propanediol over Ru-based catalysts. ChemCatChem 2018, 10, 810–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.; Kondrat, S.A.; Jones, D.R.; Schoenmakers, D.; Edwards, J.K.; Lu, L.; Yeo, B.R.; Wells, P.P.; Gibson, E.K.; Morgan, D.J.; et al. Ruthenium nanoparticles supported on carbon—an active catalyst for the hydrogenation of lactic acid to 1,2-propanediol. ACS Catal. 2015, 5, 5047–5059. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol to 1,2-propanediol over Cu-based catalysts: A short review. Catal. Today 2020, 355, 84–95. [Google Scholar] [CrossRef]
- Marchesan, A.N.; Oncken, M.P.; Maciel Filho, R.; Wolf Maciel, M.R. A roadmap for renewable C2–C3 glycols production: A process engineering approach. Green Chem. 2019, 21, 5168–5194. [Google Scholar] [CrossRef]
- Kindler, T.O.; Alberti, C.; Fedorenko, E.; Santangelo, N.; Enthaler, S. Ruthenium-catalyzed hydrogenative degradation of end-of-life poly(lactide) to produce 1,2-propanediol as platform chemical. ChemistryOpen 2020, 9, 401–404. [Google Scholar] [CrossRef]
- Stefan, W.; Jasmine, I.; Klankermayer, J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts. Sci. Adv. 2018, 4, eaat9669. [Google Scholar]
- Fernandes, A.C. Reductive depolymerization of plastic waste catalyzed by Zn(OAc)2·2H2O. ChemSusChem 2021, 14, 4228–4233. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and bio-based solvents. Top. Curr. Chem. 2018, 376, 18. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.; Zheng, H.; Du, M.; Li, Y. Vapor-phase hydrogenolysis of biomass-derived lactate to 1,2-propanediol over supported metal catalysts. Appl. Catal. A Gen. 2008, 349, 204–211. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandao, G.C.; da Silva, E.G.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Carné Sánchez, A.; Collinson, S.R. The selective recycling of mixed plastic waste of polylactic acid and polyethylene terephthalate by control of process conditions. Eur. Polym. J. 2011, 47, 1970–1976. [Google Scholar] [CrossRef] [Green Version]
- Piemonte, V.; Sabatini, S.; Gironi, F. Chemical recycling of PLA: A great opportunity towards the sustainable development? J. Polym. Environ. 2013, 21, 640–647. [Google Scholar] [CrossRef]
- Xue, J.; Cui, F.; Huang, Z.; Zuo, J.; Chen, J.; Xia, C. Effect of metal additives on structure and properties of a Co/SiO2 hydrogenation catalyst. Chin. J. Catal. 2012, 33, 1642–1649. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, X.; Han, J.; Zhu, X.; Ge, Q. Effect of calcination and metal loading on the characteristics of Co/NaY catalyst for liquid-phase hydrogenation of ethyl lactate to 1,2-propanediol. Micropor. Mesopor. Mat. 2016, 233, 184–193. [Google Scholar] [CrossRef]
- Zhang, S.; Huo, Z.; Ren, D.; Luo, J.; Fu, J.; Li, L.; Jin, F. Catalytic conversion of ethyl lactate to 1,2-propanediol over CuO. Chin. J. Chem. Eng. 2016, 24, 126–131. [Google Scholar] [CrossRef]
- Kasinathan, P.; Yoon, J.W.; Hwang, D.W.; Lee, U.H.; Hwang, J.S.; Hwang, Y.K.; Chang, J.S. Vapor-phase hydrogenation of ethyl lactate over copper–silica nanocomposites. Appl. Catal. A Gen. 2013, 451, 236–242. [Google Scholar] [CrossRef]
- Luo, G.; Yan, S.; Qiao, M.; Zhuang, J.; Fan, K. Effect of tin on Ru-B/γ-Al2O3 catalyst for the hydrogenation of ethyl lactate to 1,2-propanediol. Appl. Catal. A Gen. 2004, 275, 95–102. [Google Scholar] [CrossRef]
- Liu, K.; Litke, A.; Su, Y.; Van Campenhout, B.G.; Pidko, E.A.; Hensen, E.J. Photocatalytic decarboxylation of lactic acid by Pt/TiO2. Chem. Commun. 2016, 52, 11634–11637. [Google Scholar] [CrossRef]
- Dong, H.; Esser-Kahn, A.P.; Thakre, P.R.; Patrick, J.F.; Sottos, N.R.; White, S.R.; Moore, J.S. Chemical treatment of poly(lactic acid) fibers to enhance the rate of thermal depolymerization. ACS Appl. Mater. Interface 2012, 4, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Feng, S.; Bian, X.; Li, G.; Chen, X. Pyrolysis mechanism of poly(lactic acid) for giving lactide under the catalysis of tin. Polym. Degrad. Stab. 2018, 157, 212–223. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, J.; Huang, H.; Xiong, S.; Gao, J.; Zhang, J.; Feng, S. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2019, 175, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Ebrahimi, P.; Hadianamrei, R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm. Dev. Technol. 2014, 19, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Naik, J.B. The impact of preparation parameters on sustained release aceclofenac microspheres: A design of experiments. Adv. Powder Technol. 2015, 26, 244–252. [Google Scholar] [CrossRef]
- Somsunan, R.; Mainoiy, N. Isothermal and non-isothermal crystallization kinetics of PLA/PBS blends with talc as nucleating agent. J. Therm. Anal. Calorim. 2019, 139, 1941–1948. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interface 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Terroba-Delicado, E.; Fiori, S.; Gomez-Caturla, J.; Montanes, N.; Sanchez-Nacher, L.; Torres-Giner, S. Valorization of liquor waste derived spent coffee grains for the development of injection-molded polylactide pieces of interest as disposable food packaging and serving materials. Foods 2022, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Pérez-Camargo, R.A.; Fürst, C.; Kucharczyk, P.; Müller, A.J. Nucleating efficiency and thermal stability of industrial non-purified lignins and ultrafine talc in poly(lactic acid) (PLA). Polym. Degrad. Stab. 2017, 142, 244–254. [Google Scholar] [CrossRef]
- Pandhare, N.; Pudi, S.M.; Mondal, S.; Pareta, K.; Kumar, M.; Biswas, P. Development of Kinetic Model for Hydrogenolysis of Glycerol over Cu/MgO Catalyst in a Slurry Reactor. Ind. Eng. Chem. Res. 2017, 57, 101–110. [Google Scholar] [CrossRef]
- Kim, N.D.; Park, J.R.; Park, D.S.; Kwak, B.K.; Yi, J. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1,2-propanediol. Green Chem. 2012, 14, 2638–2646. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Catalytic production of 1,2-propanediol from glycerol in bio-ethanol solvent. Bioresour. Technol. 2012, 104, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B Environ. 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Ardila, A.N.; Sánchez-Castillo, M.A.; Zepeda, T.A.; Villa, A.L.; Fuentes, G.A. Glycerol hydrodeoxygenation to 1,2-propanediol catalyzed by CuPd/TiO2-Na. Appl. Catal. B Environ. 2017, 219, 658–671. [Google Scholar] [CrossRef]
- Wang, X.; Meng, L.; Wu, F.; Jiang, Y.; Wang, L.; Mu, X. Efficient conversion of microcrystalline cellulose to 1,2-alkanediols over supported Ni catalysts. Green Chem. 2012, 14, 758–765. [Google Scholar] [CrossRef]
- Cortright, R.; Sanchez-Castillo, M.; Dumesic, J. Conversion of biomass to 1,2-propanediol by selective catalytic hydrogenation of lactic acid over silica-supported copper. Appl. Catal. B Environ. 2002, 39, 353–359. [Google Scholar] [CrossRef]
- Ma, X.; Sun, D.; Zhao, F.; Du, C. Liquid phase hydrogenation of biomass-derived ethyl lactate to propane-1,2-diol over a highly active CoB amorphous catalyst. Catal. Commun. 2015, 60, 124–128. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Jia, Y.; Wang, J.; Liu, D.; Qin, R. Hydrogenation of ethyl lactate over ruthenium catalysts in an additive-free catalytic system. React. Kinet. Catal. Lett. 2011, 104, 89–97. [Google Scholar] [CrossRef]
- Balaraman, E.; Fogler, E.; Milstein, D. Efficient hydrogenation of biomass-derived cyclic di-esters to 1,2-diols. Chem. Commun. 2011, 48, 1111–1113. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, K.; Qin, L.; Tan, Z.; Huang, S.; Duan, P.; Kang, S. One-Pot Tandem Alcoholysis-Hydrogenation of Polylactic Acid to 1,2-Propanediol. Polymers 2023, 15, 413. https://doi.org/10.3390/polym15020413
Xu J, Zhou K, Qin L, Tan Z, Huang S, Duan P, Kang S. One-Pot Tandem Alcoholysis-Hydrogenation of Polylactic Acid to 1,2-Propanediol. Polymers. 2023; 15(2):413. https://doi.org/10.3390/polym15020413
Chicago/Turabian StyleXu, Jialin, Kuo Zhou, Linlin Qin, Zaiming Tan, Shijing Huang, Peigao Duan, and Shimin Kang. 2023. "One-Pot Tandem Alcoholysis-Hydrogenation of Polylactic Acid to 1,2-Propanediol" Polymers 15, no. 2: 413. https://doi.org/10.3390/polym15020413
APA StyleXu, J., Zhou, K., Qin, L., Tan, Z., Huang, S., Duan, P., & Kang, S. (2023). One-Pot Tandem Alcoholysis-Hydrogenation of Polylactic Acid to 1,2-Propanediol. Polymers, 15(2), 413. https://doi.org/10.3390/polym15020413