Synthesis and Properties of Modified Biodegradable Polymers Based on Caprolactone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Macro-Initiator Synthesis-Aminopropyl-Terminated Polydimethylsiloxane (APDMS-1)
2.4. Preparation of the Aminopropyl-Terminated Polydimethylsiloxane-Poly(ɛ-caprolactone) Copolymers: PDMS-PCL-1 and PDMS-PCL-2
3. Results and Discussion
3.1. Structural Characterization
3.1.1. Fourier Transform Infrared (FTIR) Analysis
3.1.2. EDX Elemental Analysis
3.2. Surface Morphology
3.2.1. Scanning Electron Microscopy Images of PDMS-PCL-1 and PDMS-PCL-2 Copolymers
3.2.2. Atomic Force Microscopy (AFM) Investigations
3.2.3. Thermal Properties of the PDMS-PCL Copolymers
3.2.4. Differential Scanning Calorimetry (DSC) Analysis
3.2.5. Contact Angle Measurement
3.2.6. Dynamic Vapor Sorption Analysis/Water Vapor Behavior
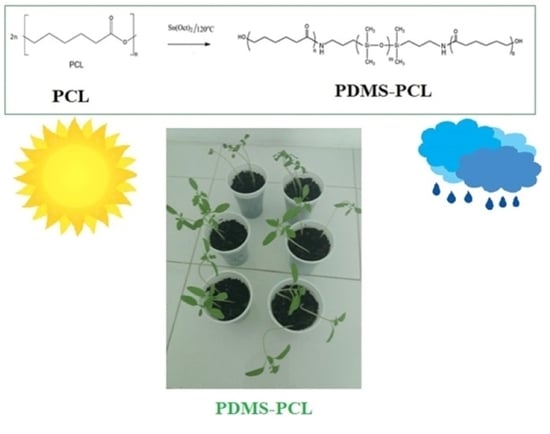
3.2.7. Biological Stability: Total Chlorophyll Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cingolani, A.; Caimi, T.S.; Casalini Klaue, A.; Sponchioni, M.; Rossi, F.; Giuseppe, P. A Methodologic Approach for the Selection of Bio-Resorbable Polymers in the Development of Medical Devices: The Case of Poly (L-lactide-co-ε-caprolactone). Polymers 2018, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Kuang, L.; Del Ponte, M.; Chain, C.; Deng, M. Design and Fabrication of Nanocomposites for Musculoskeletal Tissue Regeneration, Nanocomposites for Musculoskeletal Tissue Regeneration; Matthew Deans, Purdue University: West Lafayette, IN, USA, 2016; pp. 3–29. [Google Scholar]
- Öztürk, T.; Kılıçlıoğlu, A.; Savaş, B.; Hazer, B. Synthesis and characterization of poly (E-caprolactone-co-ethylene glycol) star-type amphiphilic copolymers by “click” chemistry and ring-opening polymerization. J. Macromol. Sci. A 2018, 55, 588–594. [Google Scholar] [CrossRef]
- Bezlepkina, K.A.; Sergey, A.M.; Vasilenko, N.G.; Muzafarov, A.M. Ring-Opening Polymerization (ROP) and Catalytic Rearrangement as a Way to Obtain Siloxane Mono- and Telechelics, as Well as Well-Organized Branching Centers: History and Prospects, Review. Polymers 2022, 14, 2408. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, E.; Isik, M.; Soz, C.K.; Yilgor, I. Synthesis and structure-property behavior of polycaprolactone-polydimethylsiloxane-polycaprolactone triblock copolymers. Polymers 2016, 83, 138–153. [Google Scholar] [CrossRef]
- Song, L.; Ahmed, M.F.; Li, Y.; Bejoy, J.; Zeng, C.; Li, Y. PCL-PDMS-PCL Copolymer-Based Microspheres Mediate Cardiovascular Differentiation from Embryonic Stem Cells. Tissue Eng. 2017, 23, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. In Encyclopedia of Polymer Science and Technology; Herman, F.M., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2017; pp. 21–34. [Google Scholar]
- Fortună, M.E.; Ignat, M.; Asandulesa, M.; Rotaru, R.; Pricop, L.; Harabagiu, V. Improved physico-chemical properties of mesoporous carbon by functionalization with aminopropyl-polydimethylsiloxane (AP-PDMS). J. Inorg Organomet Polym Mater. 2018, 28, 2275–2287. [Google Scholar] [CrossRef]
- Böhm, P. Functional Silicones and Silicone-Containing Block Copolymers. Master’s Thesis, Universitätsbibliothek Mainz, Jakob-Welder-Weg, Mainz, Germany, 2012. [Google Scholar] [CrossRef]
- Zalewski, K.; Chyłek, Z.; Trzcinski, W.A. A Review of Polysiloxanes in Terms of Their Application in Explosives. Polymers 2021, 13, 1080. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W. Synthesis and properties of triblock copolymers containing APDMS via AGET ATRP. Poly. Bull. 2021, 68, 1815–1829. [Google Scholar] [CrossRef]
- Pavlovic, D.; Linhardt, J.G.; Kunzler, J.F.; Devon, A. Shipp, Synthesis and Characterization of PDMS-, PVP- and PS-Containing ABCBA PentablockCopolymers. Macromol. Chem. Phys. 2010, 211, 1482–1487. [Google Scholar]
- Cheng, C.; Powell, K.T.; Khoshdel, E.; Wooley, K.L. Polydimethylsiloxane- (PDMS-) Grafted Fluorocopolymers by a “Grafting through” Strategy Based on Atom Transfer Radical (Co)polymerization. Macromol 2007, 40, 7195–7207. [Google Scholar] [CrossRef]
- Reddy, S.B.M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Huan, K.; Bes, L.; Haddleton, D.M.; Khoshdel, E. Synthesisand Properties of Polydimethylsiloxane-Containing Block Copolymers via Living Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1833–1842. [Google Scholar] [CrossRef]
- Pergal, M.V.; Antic, V.V.; Govedarica, M.N.; Goðevac, D.; Ostojic, S.; Djonlagic, J. Synthesis and Characterization of Novel Urethane-Siloxane Copolymers with a High Content of PCL-PDMS-PCL Segments. J. Appl. Polym. Sci. 2011, 122, 2715–2730. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Prabhakaran, M.P.; Chan, B.Q.Y.; Liow, S.S.; Ramakrishna, S.; Loh, X.J. Biocompatible Electrically Conductive Nanofibers from Inorganic-Organic Shape Memory. Polymers 2016, 16, 30689. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ungureanu, E.; Jităreanu, D.C.; Țopa, D.C.; Harabagiu, V. Effects of Hybrid Polymeric Material Based on Polycaprolactone on the Environment. Mater 2022, 15, 4868. [Google Scholar] [CrossRef]
- Mısır, M.; Ylmaz, S.S.; Bilgin, A. Synthesis and Characterization of ABA-Type Triblock Copolymers Using Novel Bifunctional PS, PMMA, and PCL Macroinitiators Bearing p-xylene-bis(2-mercaptoethyloxy) Core. Polymers 2023, 15, 3813. [Google Scholar] [CrossRef]
- Işık, M. Synthesis of Aba Type Triblock Copolymers by Ring Opening Polymerization and Investigation of the Structure Property Behavior of the Copolymers. Master’s Thesis, Kırşehir Ahi Evran University, Kırşehir, Turkey, August 2011. [Google Scholar]
- Marta, A.E.; Slabu, C.; Covasa, M.; Motrescu, I.; Lungoci, C.; Jităreanu, D.C. Influence of Environmental Factors on Some Biochemical and Physiological Indicators in Grapevine from Copou Vineyard, Iasi, Romania. Agronomy 2023, 13, 886. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Ran, Q.; Li, B.; Sun, D.; Yin, H.; Wan, Y.; Yang, C.; Liu, Y.; Mao, Y. Using Self-Synthesized Aminopropyl-Terminated Polydimethylsiloxane to Toughen Epoxy Resin: The Role of Molecular Weight of Polydimethylsiloxane. J. Vinyl Addit. Technol. 2017, 23, 305–311. [Google Scholar] [CrossRef]
- Nistor, A.; Stiubianu, G.; Racles, R.; Cazacu, M. Evaluation of the Water Sorption Capacity of Some Polymeric Materials by Dynamic Vapour Sorption. Plast. Mater. 2011, 48, 33–37. [Google Scholar]
- Iojoiu, C.; Hamaide, T.; Harabagiu, V.; Simionescu, B.C. Modified poly(e-caprolactone)s and their use for drug-encapsulating nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 689–700. [Google Scholar] [CrossRef]
- Gordin, C.; Delaite, C.; Bistac, S.; Schuller, A.S.; Rusu, D.; Rusu, M. PDMS migration at poly(vinyl chloride)/poly(ɛ-caprolactone)/poly(ɛ-caprolactone)-b-poly(dimethylsiloxane) blends surfaces. Polym. Test. 2009, 28, 446–451. [Google Scholar] [CrossRef]












| Sample | W (%) | (nm) | BET Data * | |
|---|---|---|---|---|
| Area (m2/g) | Monolayer (g/g) | |||
| PDMS-PCL-1 | 0.7940 | 2.01 | 7.879 | 0.0022 |
| PDMS-PCL-2 | 1.8993 | 1.26 | 30.007 | 0.0085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortună, M.E.; Ungureanu, E.; Rotaru, R.; Bargan, A.; Ungureanu, O.C.; Brezuleanu, C.O.; Harabagiu, V. Synthesis and Properties of Modified Biodegradable Polymers Based on Caprolactone. Polymers 2023, 15, 4731. https://doi.org/10.3390/polym15244731
Fortună ME, Ungureanu E, Rotaru R, Bargan A, Ungureanu OC, Brezuleanu CO, Harabagiu V. Synthesis and Properties of Modified Biodegradable Polymers Based on Caprolactone. Polymers. 2023; 15(24):4731. https://doi.org/10.3390/polym15244731
Chicago/Turabian StyleFortună, Maria E., Elena Ungureanu, Răzvan Rotaru, Alexandra Bargan, Ovidiu C. Ungureanu, Carmen O. Brezuleanu, and Valeria Harabagiu. 2023. "Synthesis and Properties of Modified Biodegradable Polymers Based on Caprolactone" Polymers 15, no. 24: 4731. https://doi.org/10.3390/polym15244731
APA StyleFortună, M. E., Ungureanu, E., Rotaru, R., Bargan, A., Ungureanu, O. C., Brezuleanu, C. O., & Harabagiu, V. (2023). Synthesis and Properties of Modified Biodegradable Polymers Based on Caprolactone. Polymers, 15(24), 4731. https://doi.org/10.3390/polym15244731