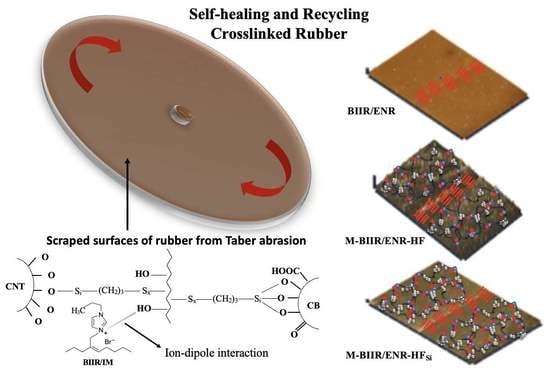
Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis(triethoxysilylpropyl)tetrasulfide Coupling Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of BIIR/NR Composites Filled with Hybrid Filler and Silane
3. Characterization
3.1. Cure Characteristics
3.2. Mechanical Properties
3.3. Morphologies
3.4. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.5. Abrasion Resistance
3.6. Dynamic Mechanical Properties
3.7. Electrical Conductivity
3.8. Filler Flocculation
4. Results and Discussion
4.1. Crosslinking Propagation
4.2. Tensile Properties and Healing Efficiency

4.3. Correlated Properties of Abrasion Ability and Dynamic Mechanical Performance
4.4. Electrical Conductivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sani, N.F.M.; Yee, H.J.; Othman, N.; Talib, A.A.; Khimi, R. Intrinsic self-healing rubber: A review and perspective of material and reinforcement. Polym. Test. 2022, 111, 107598. [Google Scholar] [CrossRef]
- Wang, X.; Liang, D.; Cheng, B. Preparation and research of intrinsic self-healing elastomers based on hydrogen and ionic bond. Compos. Sci. Technol. 2020, 193, 108127. [Google Scholar] [CrossRef]
- Lijsebetten, F.V.; Spiesschaert, Y.; Johan, M.W.; Prez, F.E.D. Reprocessing of Covalent Adaptable Polyamide Networks through Internal Catalysis and Ring-Size Effects. J. Am. Chem. Soc. 2021, 143, 15834–15844. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, X.; Zhao, F.; Song, Q.; Su, G.; Tan, Y.; Tao, Q.; Zhou, T.; Yu, Y.; Zhou, Z.; et al. Protein-Inspired self-healable Ti3C2 MXenes/rubber-based supramolecular elastomer for intelligent sensing. ACS Nano 2020, 14, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Gengying, L.; Wang, L.; Leung, C.; Hu, R.; Zhao, X.; Yan, B.; Zhou, J. Effect of styrene-butadiene rubber on the electrical properties of carbon black/cement mortar. RSC Adv. 2015, 5, 70229–70237. [Google Scholar]
- Ye, Q.; Zheng, P.; Ao, X.; Yao, D.; Lei, Z.; Deng, Y.; Wang, C. Novel multi-block conductive binder with polybutadiene for Si anodes in lithium-ion batteries. Electrochim. Acta 2019, 315, 58–66. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Mar Bernal, M.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, L. New insight into structure-property relationships of natural rubber and styrene-butadiene rubber nanocomposites filled with MWCNT. Polymer 2020, 201, 122604. [Google Scholar] [CrossRef]
- Malas, A.; Parthajit, P.; Kumar, D. Effect of expanded graphite and modified graphite flakes on the physical and thermo-mechanical properties of styrene butadiene rubber/polybutadiene rubber (SBR/BR) blends. Mater. Des. 2014, 55, 664–673. [Google Scholar] [CrossRef]
- Le, H.H.; Parsaker, M.; Sriharish, M.N.; Henning, S.; Menzel, M.; Wiessner, S.; Das, A.; Do, Q.K.; Heinrich, G.; Radusch, H.J. Effect of rubber polarity on selective wetting of carbon nanotubes in ternary blends. eXPRESS Polym. Lett. 2015, 9, 960–971. [Google Scholar] [CrossRef]
- Wemyss, A.M.; Bowen, C.; Plesse, C.; Vancaeyzeele, C.; Nguyen, G.T.M.; Vidal, F.; Wan, C. Dynamic crosslinked rubbers for a green future: A material perspective. Mater. Sci. Eng. R Rep. 2020, 141, 100561. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Lu, L.; Du, R.; Ma, W.; Cai, Y.; An, X.; Wu, H.; Luo, Q.; Xu, Q.; et al. A highly-adhesive and self-healing elastomer for bio-interfacial electrode. Adv. Funct. Mater. 2021, 31, 2006432. [Google Scholar] [CrossRef]
- Das, A.; Aladdin, S.; Böhme, F.; Suckow, M.; Basu, D.; Wießner, S.; Stöckelhuber, S.W.; Voit, B.; Heinrich, G. Ionic modification turns commercial rubber into a self-healing material. ACS Appl. Mater. Interfaces 2015, 7, 20623–20630. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Zhang, D.; Zhang, K.; Gao, C.; Wu, Y. Tough, stretchable and self- healing C-MXenes/PDMS conductive composites as sensitive strain sensors. Compos. Sci. Technol. 2021, 216, 109042. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Nakason, Y.; Kummerlöwe, C.; Vennemann, N. Effects of in-situ functionalization of carbon nanotubes with bis (triethoxysilylpropyl) tetrasulfide (TESPT) and 3-aminopropyltriethoxysilane (APTES) on properties of epoxidized natural rubber–carbon nanotube composites. Polym. Eng. Sci. 2015, 55, 2500–2510. [Google Scholar] [CrossRef]
- Chumnum, K.; Kalkornsurapranee, E.; Johns, J.; Sengloyluan, K.; Nakaramontri, Y. Combination of self-healing butyl rubber and natural rubber composites for improving the stability. Polymers 2021, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Kruželák, J.; Hudec, I. Vulcanization systems for rubber compounds based on IIR and halogenated IIR: An overview. Rubber Chem. Technol. 2018, 91, 167–183. [Google Scholar] [CrossRef]
- Tunckol, M.; Durand, J.; Serp, P. Carbon nanomaterial–ionic liquid hybrids. Carbon 2012, 50, 4303–4334. [Google Scholar] [CrossRef]
- Shubham, C.; Dhakar, G.; Kapgate, B.P.; Das, A.; Hait, S.; Gedam, R.S.; Kasilingam, R.; Das, C. Enhancing the material performance of chloroprene rubber (CR) by strategic incorporation of zirconia. Mater. Adv. 2022, 3, 2434–2446. [Google Scholar]
- Matchawet, S.; Kaesaman, A.; Vennemann, N.; Kumerlöwe, C.; Nakason, C. Effects of imidazolium ionic liquid on cure characteristics, electrical conductivity and other related properties of epoxidized natural rubber vulcanizates. Eur. Polym. J. 2017, 87, 344–359. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rao, R. Synthesis and characterization of hybrid molecular material prepared by ionic liquid and silicotungstic acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Makled, M.H.; Sheha, E.; Shanap, T.S.; El-Mansy, M.K. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2006, 12, 1–23. [Google Scholar]
- Nakaramontri, Y.; Kummerlöwe, C.; Nakason, C.; Vennemann, N. The effect of surface functionalization of carbon nanotubes on properties of natural rubber/carbon nanotube composites. Polym. Compos. 2015, 36, 2113–2122. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Kummerlöwe, C.; Nakason, C.; Vennemann, N. Influence of modified natural rubber on properties of natural rubber–carbon nanotube composites. Rubber Chem. Technol. 2015, 88, 199–218. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Kummerlöwe, C.; Vennemann, N.; Wisunthorn, S.; Pichaiyut, S.; Nakason, C. Effect of bis(triethoxysilylpropyl) tetrasulfide (TESPT) on properties of carbon nanotubes and conductive carbon black hybrid filler filled natural rubber nanocomposites. eXPRESS Polym. Lett. 2018, 12, 867–884. [Google Scholar] [CrossRef]









| Chemicals | Contents (phr *) |
|---|---|
| COMP I | |
| BIIR | 100.0 |
| IM | 5.0 |
| COMP II | |
| ENR | 100.0 |
| CNT:CB | 5.0:7.5 |
| ZnO | 5.0 |
| Stearic acid | 1.0 |
| MBTS | 1.0 |
| Sulfur | 2.5 |
| TESPT | Fix concentration ** |
| Samples | T90 | Ts1 | MH−ML |
|---|---|---|---|
| BIIR/ENR | 23.85 | 19.07 | 1.96 |
| M-BIIR/ENR | 21.26 | 8.75 | 0.33 |
| M-BIIR/ENR-HF | 3.28 | 4.25 | 0.42 |
| M-BIIR/ENR-HFSi | 2.82 | 2.25 | 0.46 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | M100 (MPa) | |||
|---|---|---|---|---|---|---|
| Before Healing | After Healing | Before Healing | After Healing | Before Healing | After Healing | |
| BIIR/ENR | 3.6 ± 0.1 | 0.5 ± 0.1 | 1515.0 ± 9.8 | 200.0 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 |
| M-BIIR/ENR | 1.9 ± 0.1 | 0.5 ± 0.1 | 1296.0 ± 10.1 | 528.0 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 |
| M-BIIR/ENR-HF | 2.2 ± 0.1 | 0.7 ± 0.1 | 1166.1 ± 9.9 | 502.0 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| M-BIIR/ENR-HFSi | 2.5 ± 0.1 | 1.2 ± 0.1 | 1098.1 ± 10.1 | 357.0 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| Wavenumber (cm−1) | Assignments |
|---|---|
| 670 | C–Br stretching vibrations |
| 870 | C–O stretching vibrations of oxirane ring |
| 1077 | Si–O-Si vibrations |
| 1110 | Si–O–C Vibrations |
| 1164 | H–C–N bending vibrations |
| 1357 | Asymmetric and symmetric C–H stretching vibrations |
| 1537 | C = O stretching vibration of Zinc stearate |
| Samples | Healing Efficiency (%) | ||
|---|---|---|---|
| Tensile Strength | Elongation at Break | M100 | |
| BIIR/ENR | 14.6 ± 1.1 | 16.4 ± 1.0 | 22.3 ± 2.2 |
| M-BIIR/ENR | 29.2 ± 1.1 | 40.7 ± 1.0 | 71.8 ± 2.2 |
| M-BIIR/ENR-HF | 31.5 ± 1.1 | 43.1 ± 1.0 | 69.4 ± 2.2 |
| M-BIIR/ENR-HFSi | 46.4 ± 1.1 | 32.5 ± 1.0 | 88.4 ± 2.2 |
| Sample | Tg from E′ | Tg from Tan δ | Tan δ at 60 °C | Tan δ at 0 °C | ||
|---|---|---|---|---|---|---|
| Tg1 | Tg2 | Tg1 | Tg2 | |||
| BIIR/ENR | −54 | −12 | −47 | −4 | 0.16 | 1.41 |
| M-BIIR/ENR | −51 | −11 | −44 | −3 | 0.26 | 1.12 |
| M-BIIR/ENR-HF | −49 | −11 | −41 | −3 | 0.25 | 1.13 |
| M-BIIR/ENR-HFSi | −47 | −10 | −38 | −2 | 0.20 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luangchuang, P.; Chumnum, K.; Kalkornsurapranee, E.; Nakaramontri, Y. Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis(triethoxysilylpropyl)tetrasulfide Coupling Agent. Polymers 2023, 15, 547. https://doi.org/10.3390/polym15030547
Luangchuang P, Chumnum K, Kalkornsurapranee E, Nakaramontri Y. Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis(triethoxysilylpropyl)tetrasulfide Coupling Agent. Polymers. 2023; 15(3):547. https://doi.org/10.3390/polym15030547
Chicago/Turabian StyleLuangchuang, Piyawadee, Kunakorn Chumnum, Ekwipoo Kalkornsurapranee, and Yeampon Nakaramontri. 2023. "Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis(triethoxysilylpropyl)tetrasulfide Coupling Agent" Polymers 15, no. 3: 547. https://doi.org/10.3390/polym15030547
APA StyleLuangchuang, P., Chumnum, K., Kalkornsurapranee, E., & Nakaramontri, Y. (2023). Increase in Properties and Self-Healing Ability of Conductive Butyl Rubber/Epoxidized Natural Rubber Composites by Using Bis(triethoxysilylpropyl)tetrasulfide Coupling Agent. Polymers, 15(3), 547. https://doi.org/10.3390/polym15030547