Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Cross-Linked PAM Aerogels
2.3. Characterization
3. Results and Discussion
3.1. Microstructure of the Cross-Linked PAM Aerogels
3.2. Mechanical Performance
3.3. Thermal Property
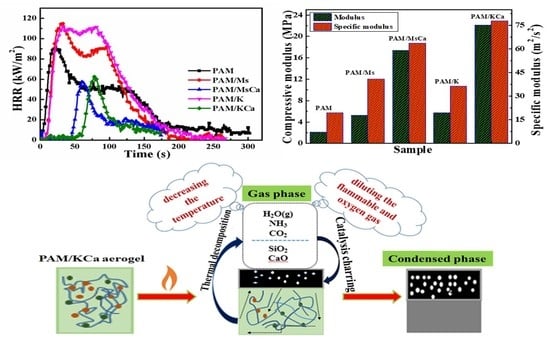
3.4. Flame Retardancy
3.5. Cone Calorimeter Test
3.6. Microstructure of Char Residue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Q.F.; Xie, R.S.; Fang, L.M.; Cai, Z.Y. Oxygen-deficient and nitrogen-doped MnO2 nanowire-reduced graphene oxide-cellulose nanofibrilaerogel electrodes for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 24407–24417. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.Y.; Li, J. Three-dimensional porous carbon aerogels from sodium carboxymethyl cellulose/poly(vinyl alcohol) composite for high-performance supercapacitors. J. Porous Mat. 2018, 25, 1679–1689. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wei, R.B.; Gu, J.W. Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption. Carbon 2018, 139, 1126–1135. [Google Scholar] [CrossRef]
- Liu, Y.N.; Su, Y.L.; Guan, J.Y. Asymmetric aerogel membranes with ultrafast water permeation for the separation of oil-in-water emulsion. ACS Appl. Mater. Interface 2018, 10, 26546–26554. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-based aerogel membrane for robust oil-in-water emulsion separation. ACS Appl. Mater. Interface 2015, 7, 24957. [Google Scholar] [CrossRef]
- She, J.; Tian, C.; Wu, Y.; Li, X.; Luo, S.; Qing, Y.; Jiang, Z. Cellulose Nanofibrils Aerogel Cross-Linked by Poly(vinyl alcohol) and Acrylic Acid for Efficient and Recycled Adsorption with Heavy Metal Ions. J. Nanosci. Nanotechnol. 2018, 18, 4167–4175. [Google Scholar] [CrossRef]
- Jiang, J.X.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Renewable, biomass-derived, honeycomblike aerogel as a robust oil absorbent with two-way reusability. ACS Sustain. Chem. Eng. 2017, 5, 10307–10316. [Google Scholar] [CrossRef]
- Zheng, Q.F.; Cai, Z.Y.; Gong, S.Q. Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Liang, Y.H.; Zhao, X.Z.; Jia, Q.Y.; Li, H.S. Preparation and microstructure of CuO-CoO-MnO/SiO2 nano-composite aerogels and xerogels as catalyst carriers. Prog. Nat. Sci-Mater. 2011, 21, 330–335. [Google Scholar] [CrossRef]
- Wu, T.; Chen, M.X.; Zhang, L.; Xu, X.Y.; Liu, Y.; Yan, J.; Wang, W.; Gao, J.P. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem. A 2013, 1, 7612–7621. [Google Scholar] [CrossRef]
- Lee, J.K.; Gould, G.L.; Rhine, W. Polyurea based aerogel for a high performance thermal insulation material. J. Sol-Gel Sci. Technol. 2009, 49, 209–220. [Google Scholar] [CrossRef]
- Wu, X.D.; Cui, S.; Wang, L.; Shao, G.F.; Jiao, J.; Shen, X.D. Advance in research of high temperature resistant aerogel used as insulation material. Mater. Rev. 2015, 294, 25–36. [Google Scholar]
- Wiener, M.; Reichenauer, G.; Braxmeier, S.; Hemberger, F.; Ebert, H.P. Carbon aerogel-based high-temperature thermal insulation. Int. J. Thermophys. 2009, 30, 1372–1385. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałeski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Liu, H.Z.; Geng, B.Y.; Chen, Y.F.; Wang, H.Y. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain. Chem. Eng. 2017, 5, 49–66. [Google Scholar] [CrossRef]
- Chen, H.-B.; Schiraldi, D.A. Flammability of Polymer/Clay Aerogel Composites: An Overview. Polym. Rev. 2018, 59, 1–24. [Google Scholar] [CrossRef]
- Liu, A.; Medina, L.; Berglund, L.A. High-Strength Nanocomposite Aerogels of Ternary Composition: Poly(vinyl alcohol), Clay, and Cellulose Nanofibrils. ACS Appl. Mater. Interfaces 2017, 9, 6453–6461. [Google Scholar] [CrossRef]
- Chen, W.S.; Yu, H.P.; Li, Q.; Liu, Y.X.; Li, J. Ultralight and highly flexible aerogels with long cellulose I nanofibers. Soft Matter 2011, 7, 10360–10368. [Google Scholar] [CrossRef]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.C.; Yong, Q.; Chen, L.; Xing, Y.J.; Hamel, J.; Zhu, H.L. Ultralight, highly thermal insulating and fire resistant aerogel by encapsulating cellulose nanofiber with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462. [Google Scholar] [CrossRef]
- Zheng, Q.; Javadi, A.; Sabo, R.; Cai, Z.Y.; Gong, S.Q. Polyvinyl alcohol (PVA)–cellulose nanofibril (CNF)–multiwalled carbon nanotube (MWCNT) hybrid organic aerogels with superior mechanical properties. RSC Adv. 2013, 3, 20816–20823. [Google Scholar] [CrossRef]
- Zhang, X.; Ziemer, K.S.; Zhang, K.; Ramirez, D.; Li, L.; Wang, S.; Hope-Weeks, K.J.; Weeks, B.L. Large-area preparation of high-quality and uniform three-dimensional graphene networks through thermal degradation of graphene oxide–nitrocellulose composites. ACS Appl. Mater. Interface 2015, 7, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interface 2011, 3, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Shen, P.; Chen, M.; Zhao, H.B.; Schiraldi, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interface 2016, 30, 32557–32564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; Chen, J.; Fan, S.; Liang, J.; Ding, L.; Chen, S. Flexible chitosan/carbon nanotubes aerogel, a robust matrix for in-situ growth and non-enzymatic biosensing applications. Sens. Actuators B Chem. 2016, 232, 750–757. [Google Scholar] [CrossRef]
- Chen, H.B.; Chiou, B.S.; Wang, Y.Z.; Schiraldi, D.A. Biodegradable pectin/clay aerogels. ACS Appl. Mater. Interface 2013, 5, 1715–1721. [Google Scholar]
- Zuo, L.Z.; Fan, W.; Zhang, Y.F.; Zhang, L.S.; Gao, W.; Huang, Y.P.; Liu, T.X. Graphene/montmorillonite hybrid synergistically reinforced polyimide composite aerogels with enhanced flame-retardant performance. Compos. Sci. Technol. 2017, 139, 57–63. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Hao, M.L.; Xu, X.; Xiong, G.P.; Li, H.; Fisher, T.S. Flyweight 3D graphene scaffolds with microinterface barrier-derived tunable thermal insulation and flame retardancy. ACS Appl. Mater. Interface 2017, 9, 14232–14241. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.H.; Dong, X.Y.; Wang, R.; Zhang, L.Q.; Wang, J.L.; Zhong, J.; Wu, L.X.; Wang, X. A pre-constructed graphene–ammonium polyphosphate aerogel (GAPPA) for efficiently enhancing the mechanical and fire-safety performances of polymers. J. Mater. Chem. A 2018, 6, 4449–4457. [Google Scholar] [CrossRef]
- Guo, W.W.; Liu, J.J.; Zhang, P.; Song, L.; Wang, X.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-friendly flame-retardant cellulose nanofibril aerogels by incorporating sodium bicarbonate. ACS Appl. Mater. Interface 2018, 10, 27407–27415. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.Y.; Zhang, X.X.; Wu, X.D.; Lu, C.H. Flame retardant, heat insulating cellulose aerogels from waste cotton fabrics by in situ formation of magnesium hydroxide nanoparticles in cellulose gel nanostructures. ACS Sustain. Chem. Eng. 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Li, H.K.; Niu, G.; Wang, J.; Zhu, D.C. Poly(vinylalcohol)/chitosan-based high-strength, fire-retardant and smoke-suppressant composite aerogels incorporating aluminum species via freeze drying. Compos. Part B 2021, 219, 108919. [Google Scholar] [CrossRef]
- Trochimczuk, A.W.; Kabay, N.; Arda, M.; Streat, M. Stabilization of solvent impregnated resins (SIRs) by coating with water soluble polymers and chemical crosslinking. React. Funct. Polym. 2004, 59, 1–7. [Google Scholar] [CrossRef]
- Shang, K.; Ye, D.D.; Kang, A.H.; Wang, Y.T.; Liao, W.; Xu, S.; Wang, Y.Z. Robust and fire retardant borate-crosslinked poly (vinyl alcohol)/montmorillonite aerogel via melt-crosslink. Polymer 2017, 131, 111. [Google Scholar] [CrossRef]
- Wu, N.J.; Niu, F.K.; Lang, W.C.; Xia, M.F. Highly efficient flame-retardant and low-smoke-toxicity poly(vinyl alcohol)/alginate/montmorillonite composite aerogels by two-step crosslinking strategy. Carbohyd. Polym. 2019, 221, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Zhao, H.B.; Degracia, K.; Han, L.X.; Sun, H.; Sun, M.Z.; Wang, Y.Z.; Schiraldi, D.A. Green approach to improving the strength and flame retardancy of poly(vinyl alcohol)/clay aerogels: Incorporating biobased gelatin. ACS Appl. Mater. Interface 2017, 9, 42258–42265. [Google Scholar] [CrossRef]
- Madyan, O.A.; Fan, M. Organic functionalization of clay aerogel and its composites through in-situ crosslinking. Appl. Clay Sci. 2019, 168, 374–381. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.Q.; Dou, L.; Yu, J.Y.; Ding, B. Ultralight and fire resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, 8925. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.J.; Li, X.T. Flame retardancy and synergistic flame retardant mechanisms of acrylonitrile-butadiene-styrene composites based on aluminum hypophosphite. Polym. Degrad. Stab. 2014, 105, 265–276. [Google Scholar] [CrossRef]
- Wu, N.J.; Lang, S.G. Flame retardancy and toughness modification of flame retardant polycarbonate/acrylonitrile-butadiene-styrene/AHP composites. Polym. Degrad. Stab. 2016, 123, 26–35. [Google Scholar] [CrossRef]








| Sample | Density /g·cm−3 | Compressive Modulus /MPa | Specific Modulus /m2·s−2 | Porosity /% |
|---|---|---|---|---|
| PAM | 0.106 ± 0.001 | 2.1 ± 0.4 | 19.5 | 99.2 |
| PAM/Ms | 0.130 ± 0.005 | 5.3 ± 0.4 | 40.9 | 98.5 |
| PAM/MsCa | 0.273 ± 0.004 | 17.4 ± 1.1 | 63.7 | 95.9 |
| PAM/K | 0.156 ± 0.002 | 5.7 ± 0.1 | 36.3 | 98.2 |
| PAM/KCa | 0.284 ± 0.003 | 22.1 ± 0.4 | 77.8 | 96.8 |
| Sample | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | dW/dT (%·min−1) | Char Yield (%) |
|---|---|---|---|---|---|
| PAM | 242.3 | 290.9 | / | 6.3 | 52.9 |
| PAM/Ms | 234.0 | 301.9 | 428.7 | 7.7 | 51.5 |
| PAM/MsCa | 157.2 | 273.8 | 448.4 | 1.8 | 64.1 |
| PAM/K | 230.5 | 242.1 | 445.2 | 3.5 | 46.2 |
| PAM/KCa | 171.9 | 265.3 | 456.9 | 2.9 | 49.6 |
| Sample | LOI (%) | t1/t2 (s/s) | UL-94 Rating |
|---|---|---|---|
| PAM | 27.0 | 6/1 | V-0 |
| PAM/Ms | 26.8 | 9/1 | V-0 |
| PAM/MsCa | 40.5 | 3/0 | V-0 |
| PAM/K | 26.5 | 8/1 | V-0 |
| PAM/KCa | 56.8 | 2/0 | V-0 |
| Sample | TTI (s) | pHRR (kW·m−2) | THR (MJ·m−2) | TSR (m2·m−2) | FPI (s·m2·kW−1) | THR/TML (MJ·m−2·g−1) | CR (%) |
|---|---|---|---|---|---|---|---|
| PAM | 6 | 94.0 | 10.3 | 21.6 | 0.06 | 1.61 | 50.0 |
| PAM/Ms | 7 | 114.8 | 10.9 | 41.1 | 0.06 | 1.88 | 47.7 |
| PAM/MsCa | 44 | 57.4 | 3.2 | 8.6 | 0.77 | 0.51 | 69.0 |
| PAM/K | 9 | 112.2 | 12.7 | 64.5 | 0.08 | 1.93 | 46.8 |
| PAM/KCa | 58 | 62.5 | 2.7 | 5.5 | 0.93 | 0.46 | 69.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Deng, S.; Wang, F.; Wang, M.; Xia, M.; Cui, H.; Jia, H. Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks. Polymers 2023, 15, 657. https://doi.org/10.3390/polym15030657
Wu N, Deng S, Wang F, Wang M, Xia M, Cui H, Jia H. Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks. Polymers. 2023; 15(3):657. https://doi.org/10.3390/polym15030657
Chicago/Turabian StyleWu, Ningjing, Shanshan Deng, Fei Wang, Mohan Wang, Mingfeng Xia, Hongli Cui, and Haoyi Jia. 2023. "Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks" Polymers 15, no. 3: 657. https://doi.org/10.3390/polym15030657
APA StyleWu, N., Deng, S., Wang, F., Wang, M., Xia, M., Cui, H., & Jia, H. (2023). Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks. Polymers, 15(3), 657. https://doi.org/10.3390/polym15030657