Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications
Abstract
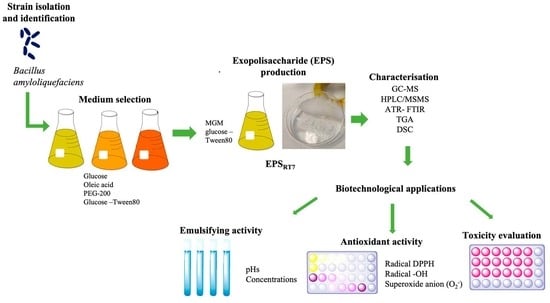
:1. Introduction
2. Materials and Methods
2.1. Chemical and Standards
2.2. Isolation of Bacterial Strain and PCR Amplification
2.3. Biodegradation, Colony-Forming Units (CFUs)/mL, pH, and EPS Production
2.3.1. Biodegradation, Colony-Forming Units (CFUs)/mL, and pH
2.3.2. EPS Production and Purification
2.4. Characterisation of EPSRT7
2.4.1. Monosaccharide Composition
2.4.2. Attenuated Total Reflectance/FT-Infrared Spectroscopy (ATR/FTIR)
2.4.3. Thermogravimetric (TGA) and Differential Scanning Calorimetric (DSC) Analysis
2.5. Emulsifying Activity Assesment
2.6. Antioxidant Activity Assesments
2.6.1. DPPH Radical Scavenging Activity
2.6.2. OH Radical Scavenging Activity
2.6.3. O2− Scavenging Activity
2.7. Exopolysaccharide Toxicity Evaluation
2.7.1. Cell Culture
2.7.2. Cytotoxicity Assay
2.8. Evaluation of Antioxidant Ability on HeLa Cells
2.8.1. Injury Inducement Model
2.8.2. Evaluation of Protective EPSRT7 Effect on HeLa Cells from Oxidative Stress
2.9. Statistical Analysis
3. Results and Discussion
3.1. Bacterial Identification, Biodegradation, and EPS Production
3.2. EPSRT7 Compositional Analysis and Characterisation
3.2.1. GC–MS Analysis for EPSRT7
3.2.2. ATR–FTIR Analysis for EPSRT7
3.2.3. Characterisation of the Thermal Properties of EPSRT7
3.3. Biotechnological Applications
3.3.1. Emulsifying Activity
3.3.2. Antioxidant Effect
3.3.3. Toxicity Evaluation and Antioxidant Ability at the Cellular Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-Based Remediation as an Effective Approach for Removal of Environmental Pollutants—A Review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Meliani, A. Enhancement of Hydrocarbons Degradation by Use of Pseudomonas Biosurfactants and Biofilms. J. Pet. Environ. Biotechnol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current Perspective of Sustainable Surfactants Based on Renewable Building Blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Venhuis, S.H.; Mehrvar, M. Health Effects, Environmental Impacts, and Photochemical Degradation of Selected Surfactants in Water. Int. J. Photoenergy 2004, 6, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Shiu, R.F.; Lee, C.L. Effects of Anthropogenic Surfactants on the Conversion of Marine Dissolved Organic Carbon and Microgels. Mar. Pollut. Bull. 2017, 117, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Rehman, R.; Ali, M.I.; Ali, N.; Badshah, M.; Iqbal, M.; Jamal, A.; Huang, Z. Crude Oil Biodegradation Potential of Biosurfactant-Producing Pseudomonas aeruginosa and Meyerozyma sp. J. Hazard. Mater. 2021, 418, 126276. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential Commercial Applications of Microbial Surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Couto, M.R.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Teixeira, J.A.; Rodrigues, L.R. Sustainable Exopolysaccharide Production by Rhizobium viscosum CECT908 Using Corn Steep Liquor and Sugarcane Molasses as Sole Substrates. Polymers 2023, 15, 20. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Alves, V.D.; Reis, M.A.M. Advances in Bacterial Exopolysaccharides: From Production to Biotechnological Applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ghahari, S.; Ghahari, S. Microbial Rejuvenation of Polluted Environment; Springer: Singapore, 2021; Volume 3, ISBN 9789811574559. [Google Scholar]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del López-Cuellar, M.R.; Rodríguez-Hernández, A.I. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for the Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Maughan, H.; Van der Auwera, G. Bacillus Taxonomy in the Genomic Era Finds Phenotypes to Be Essential Though Often Misleading. Infect. Genet. Evol. 2011, 11, 789–797. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Extremophiles and Extreme Environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, Bioemulsifiers and Exopolysaccharides from Marine Microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- Song, B.; Zhu, W.; Song, R.; Yan, F.; Wang, Y. Exopolysaccharide from Bacillus vallismortis WF4 as an Emulsifier for Antifungal and Antipruritic Peppermint Oil Emulsion. Int. J. Biol. Macromol. 2019, 125, 436–444. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Joulak, I.; Casillo, A.; Lanzetta, R.; Corsaro, M.M.; Gharsallaoui, A.; Attia, H. Potential Biotechnological Properties of an Exopolysaccharide Produced by Newly Isolated Bacillus tequilensis-GM from Spontaneously Fermented Goat Milk. LWT 2019, 105, 135–141. [Google Scholar] [CrossRef]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I.M. Production and Characterization of a Glycolipid Biosurfactant from Bacillus megaterium Using Economically Cheaper Sources. World J. Microbiol. Biotechnol. 2008, 24, 917–925. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Wu, R.; Li, P. Rheological, Emulsifying and Thermostability Properties of Two Exopolysaccharides Produced by Bacillus amyloliquefaciens LPL061. Carbohydr. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Manca, M.C.; Lama, L.; Gambacorta, A.; Maugeri, T.; Gugliandolo, C.; Caccamo, D. A Thermophilic Bacillus Isolated from an Eolian Shallow Hydrothermal Vent, Able to Produce Exopolysaccharides. Syst. Appl. Microbiol. 2000, 23, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Binmad, S.; Numnuam, A.; Kaewtatip, K.; Kantachote, D.; Tantirungkij, M. Characterization of Novel Extracellular Polymeric Substances Produced by Bacillus velezensis P1 for Potential Biotechnological Applications. Polym. Adv. Technol. 2022, 33, 2470–2479. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, G.; Wang, F.; Zhao, H.; Wei, Y.; Liu, L.; Zhang, H. Extraction, Characterization, Antioxidant Activity and Rheological Behavior of a Polysaccharide Produced by the Extremely Salt Tolerant Bacillus subtilis LR-1. LWT 2022, 162, 113413. [Google Scholar] [CrossRef]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Kaveriyappan Govindarajan, R.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and Characterization of Exopolysaccharide Produced by Bacillus aerophilus Rk1 and Its in Vitro Antioxidant Activities. J. King Saud Univ.—Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and Immunoregulatory Effect of a Novel Exopolysaccharide from a Marine Thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, E.; Bello-Morales, R.; López-Guerrero, J.A.; Poveda, A.; Jiménez-Barbero, J.; Gironès, N.; Abrusci, C. Isolation and Characterization of an Exopolymer Produced by Bacillus licheniformis: In Vitro Antiviral Activity against Enveloped Viruses. Carbohydr. Polym. 2020, 248, 116737. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A Novel EPS-Producing Strain of Bacillus licheniformis Isolated from a Shallow Vent Off Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Tween 80 Surfactant-Enhanced Bioremediation: Toward a Solution to the Soil Contamination by Hydrophobic Organic Compounds. Crit. Rev. Biotechnol. 2018, 38, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Gurugama, P.; Harper, V.; Dymond, T.; Ewan, P.; Nasser, S. The Polysorbate Containing AstraZeneca COVID-19 Vaccine Is Tolerated by Polyethylene Glycol (PEG) Allergic Patients. Clin. Exp. Allergy 2022, 52, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Abrusci, C.; Martín-González, A.; Del Amo, A.; Catalina, F.; Collado, J.; Platas, G. Isolation and Identification of Bacteria and Fungi from Cinematographic Films. Int. Biodeterior. Biodegrad. 2005, 56, 58–68. [Google Scholar] [CrossRef]
- Orphan, V.J.; Hinrichs, K.U.; Ussler, W.; Paull, C.K.; Taylor, L.T.; Sylva, S.P.; Hayes, J.M.; Delong, E.F. Comparative Analysis of Methane-Oxidizing Archaea and Sulfate-Reducing Bacteria in Anoxic Marine Sediments. Appl. Environ. Microbiol. 2001, 67, 1922–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, S.S.; Whan, V.; Davis, G.P.; Byrne, K.; Hetzel, D.J.S.; Preston, N. The Development and Application of Genetic Markers for the Kuruma Prawn Penaeus japonicus. Aquaculture 1999, 173, 19–32. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of Photo-Degraded Mulching Films Based on Polyethylenes and Stearates of Calcium and Iron as pro-Oxidant Additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Abrusci, C.; Marquina, D.; Del Amo, A.; Catalina, F. Biodegradation of Cinematographic Gelatin Emulsion by Bacteria and Filamentous Fungi Using Indirect Impedance Technique. Int. Biodeterior. Biodegrad. 2007, 60, 137–143. [Google Scholar] [CrossRef]
- Tada, H.; Shiho, O.; Kuroshima, K.; Koyama, M.; Tsukamoto, K. An Improved Colorimetric Assay for Interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R.; Wen, X.; Matin, P. Antioxidant and Cytotoxic Activities of Exopolysaccharides from Alcaligenes faecalis Species Isolated from the Marine Environment of Mauritius. J. Polym. Environ. 2022, 30, 1462–1477. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential Applications of an Exopolysaccharide Produced by Bacillus xiamenensis RT6 Isolated from an Acidic Environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Corrales, T.; Pablos, J.L.; Marin, I.; Abrusci, C. New Blends of Ethylene-Butyl Acrylate Copolymers with Thermoplastic Starch. Characterization and Bacterial Biodegradation. Carbohydr. Polym. 2016, 149, 68–76. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Sanchez-León, E.; Abrusci, C. Photodegradation and Biodegradation Under Thermophile Conditions of Mulching Films Based on Poly(Butylene Adipate-Co-Terephthalate) and Its Blend with Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 352–363. [Google Scholar] [CrossRef]
- Meneghine, A.K.; Moretto, C.; Castellane, T.C.L.; Carareto Alves, L.M. Production, Characterization and Bioemulsifying Activity of an Exopolysaccharide Produced by Sphingomonas sp. Isolated from Freshwater. J. Polym. Environ. 2017, 25, 1080–1086. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Najafpour-Darzi, G.; Morowvat, M.H.; Ghasemi, Y. Eexopolysaccharide Production of Pantoea sp. BCCS 001 GH: Physical Characterizations, Emulsification, and Antioxidant Activities. Int. J. Biol. Macromol. 2018, 118, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, B.; Prasad, B.; Rai, A.K.; Velappan, S.P.; Subbanna, M.N.; Narayan, B. In Vitro Antioxidant and Antibacterial Properties of Hydrolysed Proteins of Delimed Tannery Fleshings: Comparison of Acid Hydrolysis and Fermentation Methods. Biodegradation 2011, 22, 287–295. [Google Scholar] [CrossRef]
- Morro, A.; Abrusci, C.; Pablos, J.L.; Marín, I.; García, F.C.; García, J.M. Inherent Antibacterial Activity and in Vitro Biocompatibility of Hydrophilic Polymer Film Containing Chemically Anchored Sulfadiazine Moieties. Eur. Polym. J. 2017, 91, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Blanco, C.; Huang-Lin, E.; Abrusci, C. Characterization, Biodegradation and Cytotoxicity of Thermoplastic Starch and Ethylene-Vinyl Alcohol Copolymer Blends. Carbohydr. Polym. 2022, 298, 120085. [Google Scholar] [CrossRef]
- Amils, R.; Fernández-Remolar, D. The IPBSL Team Río Tinto: A Geochemical and Mineralogical Terrestrial Analogue of Mars. Life 2014, 4, 511–534. [Google Scholar] [CrossRef] [Green Version]
- Wongbunmak, A.; Khiawjan, S.; Suphantharika, M.; Pongtharangkul, T. BTEX Biodegradation by Bacillus amyloliquefaciens Subsp. plantarum W1 and Its Proposed BTEX Biodegradation Pathways. Sci. Rep. 2020, 10, 17408. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Niu, S.; Wang, L.; Lin, S.; Wang, C.; Ye, W.; Yan, C. Study of Phenol Biodegradation Using Bacillus amyloliquefaciens Strain WJDB-1 Immobilized in Alginate-Chitosan-Alginate (ACA) Microcapsules by Electrochemical Method. Biodegradation 2012, 23, 209–219. [Google Scholar] [CrossRef]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with Bacteria in Floating Treatment Wetlands Positively Modulates the Phytoremediation of Oil Field Wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Rao, B.P.; Kasirajan, S.; Mandal, A.B.; Sudharsan, K.; Sekaran, R.C.H.G.; Mandal, A.B. Characterization of Exopolysaccharide from Bacillus amyloliquefaciens BPRGS for Its Bioflocculant Activity. Artic. Int. J. Sci. Eng. Res. 2013, 4, 1696–1704. [Google Scholar]
- Li, S.; Fei, X.; Cao, L.; Chi, Y. Insights into the Effects of Carbon Source on Sequencing Batch Reactors: Performance, Quorum Sensing and Microbial Community. Sci. Total Environ. 2019, 691, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Sugavanam, R.; Ramakrishnan, B.; Dutta, A.; Varghese, E.; Nain, L.; Banerjee, T.; Singh, N. Free and Immobilized Microbial Culture–Mediated Crude Oil Degradation and Microbial Diversity Changes Through Taxonomic and Functional Markers in a Sandy Loam Soil. Front. Environ. Sci. 2022, 9, 794303. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Cui, H.; Qiao, J.; Guo, D.; Wang, B.; Li, X.; Huang, H. How Humic Acid and Tween80 Improve the Phenanthrene Biodegradation Efficiency: Insight from Cellular Characteristics and Quantitative Proteomics. J. Hazard. Mater. 2022, 421, 126685. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, X.; Xiao, Y.; Kong, D.; Li, Y.; Wang, H. Tween 20 Regulate the Function and Structure of Transmembrane Proteins of Bacillus cereus: Promoting Transmembrane Transport of Fluoranthene. J. Hazard. Mater. 2021, 403, 123707. [Google Scholar] [CrossRef]
- Ma, P.; Li, Y.; Miao, C.; Sun, Y.; Liu, C.; Li, H. Production of Tween 80-Inducing Esterase by Acinetobacter sp. B1 Using Response Surface Methodology. Microbiol. Biotechnol. Lett. 2019, 47, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, Y.; Zhang, Y.; He, J.; Xie, Y. Enhanced Exopolysaccharide Production in Submerged Fermentation of Ganoderma lucidum by Tween 80 Supplementation. Bioprocess Biosyst. Eng. 2021, 44, 47–56. [Google Scholar] [CrossRef]
- Deka, P.; Goswami, G.; Das, P.; Gautom, T.; Chowdhury, N.; Boro, R.C.; Barooah, M. Bacterial Exopolysaccharide Promotes Acid Tolerance in Bacillus amyloliquefaciens and Improves Soil Aggregation. Mol. Biol. Rep. 2019, 46, 1079–1091. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Soumya, M.P.; Sasikumar, K.; Pandey, A.; Nampoothiri, K.M. Cassava Starch Hydrolysate as Sustainable Carbon Source for Exopolysaccharide Production by Lactobacillus plantarum. Bioresour. Technol. Rep. 2019, 6, 85–88. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An Overview of the Functionality of Exopolysaccharides Produced by Lactic Acid Bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Jiang, Y.Y.; Zhao, X.; Hao, X.N.; Li, L.; Yang, Z.N. Characterization and Antioxidant Activity of the Exopolysaccharide Produced by Bacillus amyloliquefaciens GSBa-1. J. Microbiol. Biotechnol. 2018, 28, 1282–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, Characterization and Biological Activities of Acidic Exopolysaccharide from Marine Bacillus amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 652–662. [Google Scholar] [CrossRef]
- Cai, G.; Liu, Y.; Li, X.; Lu, J. New Levan-Type Exopolysaccharide from Bacillus amyloliquefaciens as an Antiadhesive Agent against Enterotoxigenic Escherichia coli. J. Agric. Food Chem. 2019, 67, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two Novel Exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and Effect on Oxidative Stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.H.; Huang, Y.Y.; Hu, J.S.; Yu, J.J.; Zhou, Q.Y.; Liu, D.M. Exopolysaccharides from Bacillus amyloliquefaciens DMBA-K4 Ameliorate Dextran Sodium Sulfate-Induced Colitis via Gut Microbiota Modulation. J. Funct. Foods 2020, 75, 104212. [Google Scholar] [CrossRef]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, Purification and Structural Study of an Exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Thakham, N.; Thaweesak, S.; Teerakulkittipong, N.; Traiosot, N.; Kaikaew, A.; Lirio, G.A.; Jangiam, W. Structural Characterization of Functional Ingredient Levan Synthesized by Bacillus siamensis Isolated from Traditional Fermented Food in Thailand. Int. J. Food Sci. 2020, 2020, 7352484. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Jayaraman Uthaya, C.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, Compositional Analysis, and Characterization of Exopolysaccharides Produced by Multi-Metal Resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. A Novel Exopolysaccharide from Marine Bacterium Pantoea sp. YU16-S3 Accelerates Cutaneous Wound Healing through Wnt/β-Catenin Pathway. Carbohydr. Polym. 2020, 238, 116191. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Esposito, G.; Hunashal, Y.; Obaid, R.S.; Hamed, F. Characterization, Bioactivities, and Rheological Properties of Exopolysaccharide Produced by Novel Probiotic Lactobacillus plantarum C70 Isolated from Camel Milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Taylan, O.; Yilmaz, M.T.; Dertli, E. Partial Characterization of a Levan Type Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides Showing Immunostimulatory and Antioxidant Activities. Int. J. Biol. Macromol. 2019, 136, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nampoothiri, K.M. Production, Purification and Structural Characterization of an Exopolysaccharide Produced by a Probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef]
- Gangalla, R.; Gattu, S.; Palaniappan, S.; Ahamed, M.; Macha, B.; Thampu, R.K.; Fais, A.; Cincotti, A.; Gatto, G.; Dama, M.; et al. Structural Characterisation and Assessment of the Novel Bacillus amyloliquefaciens RK3 Exopolysaccharide on the Improvement of Cognitive Function in Alzheimer’s Disease Mice. Polymers 2021, 13, 2842. [Google Scholar] [CrossRef] [PubMed]
- Jenny Angel, S.; Vidyadharani, G.; Santhosh, S.; Dhandapani, R. Optimization and Characterisation of Thermo Stable Exopolysaccharide Produced from Bacillus licheniformis WSF-1 Strain. J. Polym. Environ. 2018, 26, 3824–3833. [Google Scholar] [CrossRef]
- Song, B.; Song, R.; Cheng, M.; Chu, H.; Yan, F.; Wang, Y. Preparation of Calcipotriol Emulsion Using Bacterial Exopolysaccharides as Emulsifier for Percutaneous Treatment of Psoriasis Vulgaris. Int. J. Mol. Sci. 2020, 21, 77. [Google Scholar] [CrossRef] [Green Version]
- Ben Ayed, H.; Jemil, N.; Maalej, H.; Bayoudh, A.; Hmidet, N.; Nasri, M. Enhancement of Solubilization and Biodegradation of Diesel Oil by Biosurfactant from Bacillus amyloliquefaciens An6. Int. Biodeterior. Biodegrad. 2015, 99, 8–14. [Google Scholar] [CrossRef]
- Bouallegue, A.; Chaari, F.; Casillo, A.; Corsaro, M.M.; Bachoual, R.; Ellouz-Chaabouni, S. Levan Produced by Bacillus subtilis AF17: Thermal, Functional and Rheological Properties. J. Food Meas. Charact. 2022, 16, 440–447. [Google Scholar] [CrossRef]
- Kanmani, P.; Satish kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Production and Purification of a Novel Exopolysaccharide from Lactic Acid Bacterium Streptococcus phocae PI80 and Its Functional Characteristics Activity in Vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef]
- Kodali, V.P.; Das, S.; Sen, R. An Exopolysaccharide from a Probiotic: Biosynthesis Dynamics, Composition and Emulsifying Activity. Food Res. Int. 2009, 42, 695–699. [Google Scholar] [CrossRef]
- Hu, X.; Li, F.; Zhang, X.; Pan, Y. The Structure, Characterization and Dual-Activity of Exopolysaccharide Produced by Bacillus enclensis AP-4 from Deep-Sea Sediments. Front. Mar. Sci. 2022, 9, 976543. [Google Scholar] [CrossRef]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of Antioxidant, Antibacterial and Cytotoxicity Activities of Exopolysaccharide from Enterococcus Strains Isolated from Traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods That Define Mechanism of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen Vu, T.H.; Quach, N.T.; Nguyen, N.A.; Nguyen, H.T.; Ngo, C.C.; Nguyen, T.D.; Ho, P.H.; Hoang, H.; Chu, H.H.; Phi, Q.T. Genome Mining Associated with Analysis of Structure, Antioxidant Activity Reveals the Potential Production of Levan-Rich Exopolysaccharides by Food-Derived Bacillus velezensis Vtx20. Appl. Sci. 2021, 11, 7055. [Google Scholar] [CrossRef]
- Yang, X.; Feng, J.; Zhu, Q.; Hong, R.; Li, L. A Relation between Exopolysaccharide from Lactic Acid Bacteria and Properties of Fermentation Induced Soybean Protein Gels. Polymers 2022, 14, 90. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, S.; Tu, J.H.; Yu, J.S.; Nurlatifah, A.O.; Chiu, W.C.; Su, Y.H.; Chang, H.L.; Putri, D.A.; Cheng, H.L. Exopolysaccharides of Bacillus amyloliquefaciens Modulate Glycemic Level in Mice and Promote Glucose Uptake of Cells through the Activation of Akt. Int. J. Biol. Macromol. 2020, 146, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Tu, J.H.; Yu, J.S.; Ulfa, M.Z.; Chang, J.H.; Cheng, H.L. Bacillus amyloliquefaciens Exopolysaccharide Preparation Induces Glucagon-like Peptide 1 Secretion through the Activation of Bitter Taste Receptors. Int. J. Biol. Macromol. 2021, 185, 562–571. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, S.; Li, J.; Zhou, Y.; Chen, T.; Feng, S.; Ding, C. Simultaneous Optimization of Extraction and Antioxidant Activity from Blumea laciniata and the Protective Effect on Hela Cells against Oxidative Damage. Arab. J. Chem. 2020, 13, 9231–9242. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-León, E.; Huang-Lin, E.; Amils, R.; Abrusci, C. Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers 2023, 15, 1550. https://doi.org/10.3390/polym15061550
Sánchez-León E, Huang-Lin E, Amils R, Abrusci C. Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers. 2023; 15(6):1550. https://doi.org/10.3390/polym15061550
Chicago/Turabian StyleSánchez-León, Enrique, Elisa Huang-Lin, Ricardo Amils, and Concepción Abrusci. 2023. "Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications" Polymers 15, no. 6: 1550. https://doi.org/10.3390/polym15061550
APA StyleSánchez-León, E., Huang-Lin, E., Amils, R., & Abrusci, C. (2023). Production and Characterisation of an Exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological Applications. Polymers, 15(6), 1550. https://doi.org/10.3390/polym15061550