Insight into the Molecular Weight of Hydrophobic Starch Laurate-Based Adhesives for Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
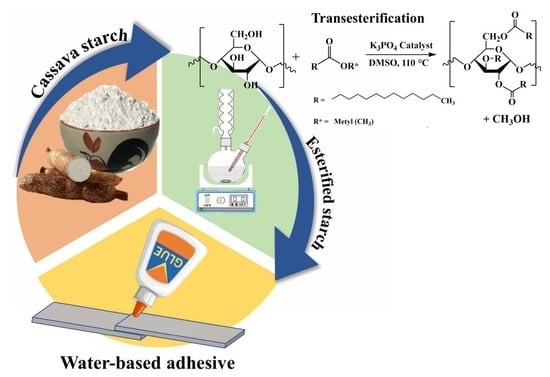
2.2.1. Cassava Starch Modification
2.2.2. Preparation of Starch-Based Adhesive
2.2.3. Characterizations of Starches
Degree of Substitution (DS)
Gel Permeation Chromatography (GPC)
Scanning Electron Microscopy (SEM)
Fourier Transform Infrared (FTIR) Spectroscopy
Raman Spectroscopy
Solubility and Swelling Power
Contact Angle Measurement
Thermal Analysis
Viscosity Measurement
Adhesive Bonding Performance
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Degree of Substitution (DS)
3.2. Gel Permeation Chromatography
3.3. Scanning Electron Microscopy (SEM)
3.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.5. Raman Spectroscopy
3.6. Water Solubility and Swelling Power of Starches
3.7. Thermal Properties of Starches
3.8. Contact Angle
3.9. Viscosity and Bond Strength
3.10. Effect of Reaction Times on the Properties of Starch Laurates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opara, I.J.; Ossi, C.D.; OkoUdu, C.O. Formulation of Cassava Starch-Based Adhesive. Int. J. Adv. Res. 2017, 5, 26–33. [Google Scholar] [CrossRef]
- Bartoszuk, K.; Kowaluk, G. Moisture Influence on Solid Wood Bonded with Modified Starch. Ann. WULS For. Wood Technol. 2022, 118, 67–73. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Zhang, B.; Qiao, D.; Pu, H.; Liu, S.; Li, L. Structural Features and Thermal Property of Propionylated Starches with Different Amylose/Amylopectin Ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Celikci, N.; Dolaz, M.; Colakoglu, A.S. An Environmentally Friendly Carton Adhesive from Acidic Hydrolysis of Waste Potato Starch. Int. J. Polym. Anal. Charact. 2021, 26, 97–110. [Google Scholar] [CrossRef]
- Lamaming, J.; Heng, N.B.; Owodunni, A.A.; Lamaming, S.Z.; Khadir, N.K.A.; Hashim, R.; Sulaiman, O.; Kassim, M.H.M.; Hussin, M.H.; Bustami, Y.; et al. Characterization of Rubberwood Particleboard Made Using Carboxymethyl Starch Mixed with Polyvinyl Alcohol as Adhesive. Compos. Part B Eng. 2020, 183, 107731. [Google Scholar] [CrossRef]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef]
- Imre, B.; Vilaplana, F. Organocatalytic Esterification of Corn Starches towards Enhanced Thermal Stability and Moisture Resistance. Green Chem. 2020, 22, 5017–5031. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, J.; Wang, Z. Preparation and Properties of Cassava Starch-Based Wood Adhesives. BioResources 2016, 11, 6756–6767. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and Properties of a Starch-Based Wood Adhesive with High Bonding Strength and Water Resistance. Carbohydr. Polym. 2015, 115, 32–57. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Wetzel, H. Synthesis and Properties of Fatty Acid Starch Esters. Carbohydr. Polym. 2013, 98, 208–216. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical Properties of Starch Adhesives Enhanced by Esterification Modification with Dodecenyl Succinic Anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Lamaming, J.; Hashim, R.; Abdulwahab Taiwo, O.F.; Hussin, M.H.; Kassim, M.H.M.; Bustami, Y.; Sulaiman, O.; Amini, M.H.M.; Hiziroglu, S. Properties of Green Particleboard Manufactured from Coconut Fiber Using a Potato Starch Based Adhesive. BioResources 2020, 15, 2279–2292. [Google Scholar] [CrossRef]
- Ojogbo, E.; Blanchard, R.; Mekonnen, T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure–Property Correlations. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2611–2622. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Nontamas, P.; Phatthanakun, R.; Chio-Srichan, S.; Soontaranon, S.; Sorndech, W.; Tongta, S. Physico-Chemical Properties and Digestibility of Native and Citrate Starches Change in Different Ways by Synchrotron Radiation. Int. J. Biol. Macromol. 2022, 207, 475–483. [Google Scholar] [CrossRef]
- Roman-Gutierrez, A.; Sabathier, J.; Guilbert, S.; Galet, L.; Cuq, B. Characterization of the Surface Hydration Properties of Wheat Flours and Flour Components by the Measurement of Contact Angle. Powder Technol. 2003, 129, 37–45. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, T.; Ahmad, S.; Cheng, J.; Guo, M. Physicochemical and Adhesive Properties, Microstructure and Storage Stability of Whey Protein-Based Paper Glue. Int. J. Adhes. Adhes. 2013, 41, 198–205. [Google Scholar] [CrossRef]
- PASW Statistics for Windows, Version 18.0; SPSS Inc.: Chicago, IL, USA, 2009.
- Gaborieau, M.; Castignolles, P. Size-Exclusion Chromatography (SEC) of Branched Polymers and Polysaccharides. Anal. Bioanal. Chem. 2011, 399, 1413–1423. [Google Scholar] [CrossRef]
- Szwengiel, A.; Kubiak, P. Molecular Dispersion of Starch as a Crucial Parameter during Size-Exclusion Chromatography. Foods 2020, 9, 204. [Google Scholar] [CrossRef]
- Lu, X.; Luo, Z.; Fu, X.; Xiao, Z. Two-Step Method of Enzymatic Synthesis of Starch Laurate in Ionic Liquids. J. Agric. Food Chem. 2013, 61, 9882–9891. [Google Scholar] [CrossRef]
- Zhao, X.F.; Peng, L.Q.; Wang, H.L.; Wang, Y.B.; Zhang, H. Environment-Friendly Urea-Oxidized Starch Adhesive with Zero Formaldehyde-Emission. Carbohydr. Polym. 2018, 181, 1112–1118. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Yan, Y.; Hu, D.; Yang, L.; Shen, R. Application of Raman Spectroscopy in Structure Analysis and Crystallinity Calculation of Corn Starch. Starch/Staerke 2015, 67, 612–619. [Google Scholar] [CrossRef]
- Wijaya, C.; Do, Q.D.; Ju, Y.H.; Santoso, S.P.; Putro, J.N.; Laysandra, L.; Soetaredjo, F.E.; Ismadji, S. Isolation and Characterization of Starch from Limnophila Aromatica. Heliyon 2019, 5, e01622. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, R.P.; Gilbert, R.G.; Fitzgerald, M.A. Structural Differences between Hot-Water-Soluble and Hot-Water-Insoluble Fractions of Starch in Waxy Rice (Oryza sativa L.). Carbohydr. Polym. 2010, 81, 524–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, T.; Hu, H.; Huang, Z.; Huang, A.; Zhu, Y.; Feng, Z.; Yang, M. A Green Technology for the Preparation of High Fatty Acid Starch Esters: Solid-Phase Synthesis of Starch Laurate Assisted by Mechanical Activation with Stirring Ball Mill as Reactor. Ind. Eng. Chem. Res. 2014, 53, 2114–2120. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G.; Koster, K.L. What Is “Unfreezable Water”, How Unfreezable Is It and How Much Is There? Cryo-Letters 2002, 23, 157–166. [Google Scholar]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Q.; Tan, H.; Gu, J.; Zhang, Y. Starch-Based Wood Adhesive. BioResources 2019, 14, 1405–1418. [Google Scholar] [CrossRef]
- Egharevba, H.O. Chemical Properties of Starch and Its Application in the Food Industry; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, B.; Wang, X.A.; Wu, Z.; Wei, W.; Aladejana, J.T.; Hou, X.; Yves, K.G.; Xie, Y.; Liu, J. Chitosan Used as a Specific Coupling Agent to Modify Starch in Preparation of Adhesive Film. J. Clean. Prod. 2020, 277, 123210. [Google Scholar] [CrossRef]
- Wang, G.; Guo, M. Property and Storage Stability of Whey Protein-Sucrose Based Safe Paper Glue. J. Appl. Polym. Sci. 2014, 131, 39710. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, J.; Singh, I.; Rakesh, P.K. Joining Behavior of Natural Fiber Reinforced Polymer Composites, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; ISBN 9780323853996. [Google Scholar]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable Panels Design Based on Modified Cassava Starch Bioadhesives and Wood Processing Byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Law, K.Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar]





| Rx. Time (h) | Water | DMSO | |||
|---|---|---|---|---|---|
| Mw, t = 9 min (Da) | Mw, t = 12–14 min (Da) | Mw, t = 26 min (Da) | Mw, t = 0.7 min (Da) | Mw, t = 29 min (Da) | |
| 0 | n.d. | 5.58 × 105 | 8.02 × 103 | 4.80 × 1011 | n.d. |
| 1 | n.d. | 4.08 × 105 | 7.95 × 103 | 4.79 × 1011 | 1.87 × 108 |
| 2 | n.d. | 3.76 × 105 | 7.98 × 103 | 4.82 × 1011 | 1.80 × 108 |
| 4 | 1.47 × 106 | 3.80 × 105 | 8.06 × 103 | 4.81 × 1011 | 1.94 × 108 |
| 6 | 1.63 × 106 | 4.45 × 105 | 8.26 × 103 | 4.81 × 1011 | 1.94 × 108 |
| 10 | 1.72 × 106 | 3.28 × 105 | 8.50 × 103 | n.d. | 1.92 × 108 |
| Rx. Time (h) | DS NMR | Gelatinization | Decomposition Temp. (°C) | Contact Angle (Degree, °) | ||
|---|---|---|---|---|---|---|
| Temp. (°C) | ΔH (J/g) | |||||
| Native starch | 0 | 0 | 65.8 | 1.13 | 290.4 | 82.2 |
| Starch laurate | 1 | 0.07 | 96.9 | 6.06 | 216.8 | 88.7 |
| 2 | 0.10 | 94.6 | 50.3 | 218.0 | 97.9 | |
| 4 | 0.13 | 95.9 | 31.8 | 218.9 | 120.5 | |
| 6 | 0.17 | 94.7 | 19.3 | 220.9 | 127.3 | |
| 10 | 0.22 | 95.2 | 9.82 | 222.4 | 132.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watcharakitti, J.; Nimnuan, J.; Krusong, K.; Nanan, S.; Smith, S.M. Insight into the Molecular Weight of Hydrophobic Starch Laurate-Based Adhesives for Paper. Polymers 2023, 15, 1754. https://doi.org/10.3390/polym15071754
Watcharakitti J, Nimnuan J, Krusong K, Nanan S, Smith SM. Insight into the Molecular Weight of Hydrophobic Starch Laurate-Based Adhesives for Paper. Polymers. 2023; 15(7):1754. https://doi.org/10.3390/polym15071754
Chicago/Turabian StyleWatcharakitti, Jidapa, Jaturavit Nimnuan, Kuakarun Krusong, Suwat Nanan, and Siwaporn Meejoo Smith. 2023. "Insight into the Molecular Weight of Hydrophobic Starch Laurate-Based Adhesives for Paper" Polymers 15, no. 7: 1754. https://doi.org/10.3390/polym15071754
APA StyleWatcharakitti, J., Nimnuan, J., Krusong, K., Nanan, S., & Smith, S. M. (2023). Insight into the Molecular Weight of Hydrophobic Starch Laurate-Based Adhesives for Paper. Polymers, 15(7), 1754. https://doi.org/10.3390/polym15071754