Bacterial Nanocellulose from Komagataeibacter Medellinensis in Fique Juice for Activated Carbons Production and Its Application for Supercapacitor Electrodes
Abstract
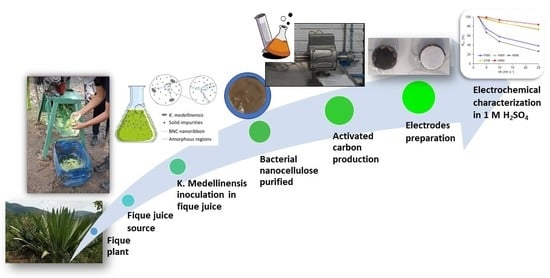
:1. Introduction
2. Materials and Methods
2.1. Chemical Activation of the Bacterial Nanocellulose
2.2. Morphological, Superficial, Porous, and Physicochemical Characterization
2.3. Electrochemical Characterization
- Electrochemical impedance spectroscopy (EIS) is a technique used to characterize the frequency response of a device. The Nyquist plot was carried out in a frequency range between 1 mHz and 100 kHz with a sinusoidal amplitude of ± 10 V. The CEIS (F g−1) is the maximum gravimetric capacitance, which was determined according to Equation (1) at the minimum frequency, and the interfacial capacitance IC (μF cm−2) is the gravimetric capacitance normalized by the specific surface area according to Equation (2).
- The cyclic voltammetry (CV) technique applies a potential perturbation (scan rate) in a specific potential range. The cyclic voltammetry curves were carried out between 0 and 0.75 V at different scan rates : 0.5, 1, 2, 5, 10, 25, and 50 mV s−1. The kinetic or diffusion limitation can be detected from CV curves [24]. The CCV (F g−1) is the gravimetric capacitance, and it was determined from the voltammetry curves according to Equation (3).
- The chronopotentiometry (CP) technique applies a constant current density and measures the potential (E) with respect to time, allowing it to determine the device’s stability. The charge and discharge curves were performed between a potential window from 0 to 0.75 V at different current densities: 0.156, 0.313, 0.625, and 0.938 A g−1. The gravimetric capacity (CCP) was calculated from the discharge curve according to Equation (5). Additionally, the best samples underwent cyclability analysis at the 0.33 A g−1 current density (5000 charge–discharge cycles).
3. Results and Discussion
3.1. Morphological Characterization of BNCAs
3.2. Superficial and Porous Characterization of BNCAs
3.3. Physico-Chemical Characterization of BNCAs
3.4. Electrochemical Characterization of BNCAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, B.H.; Tseng, C.-L. Distributed energy resources: Issues and challenges. J. Energy Eng. 2007, 133, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Geng, X.; Chen, Q.; Ren, T.-L. A better Zn-ion storage device: Recent progress for Zn-ion hybrid supercapacitors. Nano-Micro Lett. 2022, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A Brief Overview; MITRE Corporation: McLean, VA, USA, 2006. [Google Scholar]
- Ho, J.; Jow, T.R.; Boggs, S. Historical introduction to capacitor technology. IEEE Electr. Insul. Mag. 2010, 26, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Belaineh, D.; Brooke, R.; Sani, N.; Say, M.G.; Håkansson, K.M.; Engquist, I.; Berggren, M.; Edberg, J. Printable carbon-based supercapacitors reinforced with cellulose and conductive polymers. J. Energy Storage 2022, 50, 104224. [Google Scholar] [CrossRef]
- Calvo, E.; Arenillas, A.; Menendez, J.; Gonzalez, M.; Viera, J. Properties, advantages and disadvantages of materials used in supercapacitors. Afinidad 2009, 66, 380–387. [Google Scholar]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef]
- Yang, S.; Cui, Y.; Yang, G.; Zhao, S.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. ZnCl2 induced hierarchical porous carbon for zinc-ion hybrid supercapacitors. J. Power Sources 2023, 554, 232347. [Google Scholar] [CrossRef]
- Castro, C.; Delgado, F. La nanocelulosa: Propiedades y aplicaciones. Boletín IEE 2016, 40, 56–60. [Google Scholar]
- Ma, L.; Bi, Z.; Xue, Y.; Zhang, W.; Huang, Q.; Zhang, L.; Huang, Y. Bacterial cellulose: An encouraging eco-friendly nano-candidate for energy storage and energy conversion. J. Mater. Chem. A 2020, 8, 5812–5842. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Asoh, T.-A.; Li, C.; Dan, Y.; Uyama, H. Controlled preparation of interconnected 3D hierarchical porous carbons from bacterial cellulose-based composite monoliths for supercapacitors. Nanoscale 2020, 12, 15261–15274. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices-A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Boongate, C.; Phisalaphong, M. Activated carbons from bacterial cellulose by chemical activation with potassium hydroxide. In Proceedings of the 2015 International Conference on Science and Technology (TICST), Pathum Thani, Thailand, 4–6 November 2015; pp. 144–147. [Google Scholar]
- Khamkeaw, A.; Phanthang, L.; Jongsomjit, B.; Phisalaphong, M. Activated carbon derived from bacterial cellulose and its use as catalyst support for ethanol conversion to ethylene. Catal. Commun. 2019, 129, 105750. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Asavamongkolkul, T.; Perngyai, T.; Jongsomjit, B.; Phisalaphong, M. Interconnected Micro, Meso, and Macro Porous Activated Carbon from Bacterial Nanocellulose for Superior Adsorption Properties and Effective Catalytic Performance. Molecules 2020, 25, 4063. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Qian, H.; Tay, F.H.; Blaker, J.J.; Kazarian, S.G.; Bismarck, A. Bacterial cellulose as source for activated nanosized carbon for electric double layer capacitors. J. Mater. Sci. 2013, 48, 367–376. [Google Scholar] [CrossRef]
- Martínez, E.; Posada, L.; Botero, J.; Rios-Arango, J.; Zapata-Benabithe, Z.; López, S.; Molina-Ramírez, C.; Osorio, M.; Castro, C. Nata de fique: A cost-effective alternative for the large-scale production of bacterial nanocellulose. Ind. Crops Prod. 2023, 192, 116015. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Trček, J.; Zuluaga, R.; De Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.-L.; Ganan, P. Gluconacetobacter medellinensis sp. nov., cellulose-and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M. Generalization of the theory of volume filling of micropores to nonhomogeneous microporous structures. Carbon 1985, 23, 373–380. [Google Scholar] [CrossRef]
- Stoeckli, F. The name of this reference is wrong. The correct name is Water adsorption in activated carbons of various degrees of oxidation described by the Dubinin equation. Carbon 2002, 40, 969–971. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical supercapacitors for energy storage and conversion. Handb. Clean Energy Syst. 2015, 1–25. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Chen, G.; Kim, N.Y.; Ni, K.; Zeng, W.; Zhao, Y.; Tao, Z.; Ji, H.; Lee, Z.; Zhu, Y. Creating Pores on Graphene Platelets by Low-Temperature KOH Activation for Enhanced Electrochemical Performance. Small 2016, 12, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sabio, M.; Rodrıguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Viswanathan, B.; Neel, P.I.; Varadarajan, T. Methods of activation and specific applications of carbon materials. India Chennai 2009, 600, 1–160. [Google Scholar]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef] [Green Version]
- Puziy, A.; Poddubnaya, O.; Ziatdinov, A. On the chemical structure of phosphorus compounds in phosphoric acid-activated carbon. Appl. Surf. Sci. 2006, 252, 8036–8038. [Google Scholar] [CrossRef]
- Li, W.-J.; Chou, S.-L.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Significant enhancement of the cycling performance and rate capability of the P/C composite via chemical bonding (P–C). J. Mater. Chem. A 2016, 4, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, K.; Liu, Y.; Pu, L.; Chen, Z.; Deng, S. The high-performance and mechanism of P-doped activated carbon as a catalyst for air-cathode microbial fuel cells. J. Mater. Chem. A 2015, 3, 21149–21158. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, R.; Liang, Z.; Long, D.-H.; Qiao, W.-M.; Yang, J.-h.; Ling, L.-C. Impedance of carbon aerogel/activated carbon composites as electrodes of electrochemical capacitors in aprotic electrolyte. New Carbon Mater. 2007, 22, 153–158. [Google Scholar] [CrossRef]
- Ghaemi, M.; Ataherian, F.; Zolfaghari, A.; Jafari, S. Charge storage mechanism of sonochemically prepared MnO2 as supercapacitor electrode: Effects of physisorbed water and proton conduction. Electrochim. Acta 2008, 53, 4607–4614. [Google Scholar] [CrossRef]
- Qu, J.; Geng, C.; Lv, S.; Shao, G.; Ma, S.; Wu, M. Nitrogen, oxygen and phosphorus decorated porous carbons derived from shrimp shells for supercapacitors. Electrochim. Acta 2015, 176, 982–988. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Cui, L. Recent progress of mesoporous materials for high performance supercapacitors. Microporous Mesoporous Mater. 2021, 314, 110870. [Google Scholar] [CrossRef]
- Bordjiba, T.; Mohamedi, M.; Dao, L.H. Synthesis and electrochemical capacitance of binderless nanocomposite electrodes formed by dispersion of carbon nanotubes and carbon aerogels. J. Power Sources 2007, 172, 991–998. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Sun, J.; Li, K.; Zhao, S.; Zhao, D.; Wang, J.; Yang, C.; Wang, X.; Cao, B. Corncob-derived hierarchical porous activated carbon for high-performance lithium-ion capacitors. Energy Fuels 2020, 34, 16885–16892. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Yang, D.; Yang, M.; Li, H. Oxygen and nitrogen co-doped porous carbons with finely-layered schistose structure for high-rate-performance supercapacitors. Carbon 2017, 122, 538–546. [Google Scholar] [CrossRef]
- Babić, B.; Kaluđerović, B.; Vračar, L.; Krstajić, N. Characterization of carbon cryogel synthesized by sol–gel polycondensation and freeze-drying. Carbon 2004, 42, 2617–2624. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Li, Z.; Lin, N.; Xu, H.; Gao, S. Sustainable supercapacitors of nitrogen-doping porous carbon based on cellulose nanocrystals and urea. Int. J. Biol. Macromol. 2020, 164, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Xia, W.; Gong, J.; Jia, S.; Zhang, J. Nitrogen, phosphorus and sulfur co-doped pyrolyzed bacterial cellulose nanofibers for supercapacitors. Nanomaterials 2020, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, X.; Zhao, Y.; Zhou, J.; Ma, X.; Xie, E. Organic molecules assisted synthesis of carbon nanofibers with controlled surface area for high-performance supercapacitors. J. Energy Storage 2020, 31, 101653. [Google Scholar] [CrossRef]
- Fang, D.; Zhou, J.; Sheng, L.; Tang, W.; Tang, J. Juglone bonded carbon nanotubes interweaving cellulose nanofibers as self-standing membrane electrodes for flexible high energy supercapacitors. Chem. Eng. J. 2020, 396, 125325. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Chen, Z.; Deng, Z.; Fan, L.; Guo, W.; Xu, J.; Meng, Z. High-performance yarn supercapacitor based on directly twisted carbon nanotube@ bacterial cellulose membrane. Cellulose 2020, 27, 7649–7661. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, X.Y. Carbon nanofibers derived from bacterial cellulose: Surface modification by polydopamine and the use of ferrous ion as electrolyte additive for collaboratively increasing the supercapacitor performance. Appl. Surf. Sci. 2020, 519, 146252. [Google Scholar] [CrossRef]
- Wannasen, L.; Swatsitang, E.; Pinitsoontorn, S. Flexible supercapacitors based on mesoporous nanocrystalline cobalt ammonium phosphates and bacterial cellulose composite electrode. Int. J. Energy Res. 2021, 45, 3075–3088. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; Bai, Z.; Liang, G.; Liu, L.; Fang, D.; Xu, W. Conductive polypyrrole–bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org. Electron. 2013, 14, 3331–3338. [Google Scholar] [CrossRef]
- Martinez-Casillas, D.C.; Mascorro-Gutierrez, I.; Betancourt-Mendiola, M.L.; Palestino, G.; Quiroga-Gonzalez, E.; Pascoe-Sussoni, J.E.; Guillen-Lopez, A.; Muniz, J.; Cuentas-Gallegos, A.K. Residue of corncob gasification as electrode of supercapacitors: An experimental and theoretical study. Waste Biomass Valorization 2021, 12, 4123–4140. [Google Scholar] [CrossRef]










| BNCA | SBET (m2 g−1) | Dmicro (nm) | VN2 (cm3 g−1) | Vtotal,0.985 (cm3 g−1) | Vmeso (cm3 g−1) | VCO2 (cm3 g−1) |
|---|---|---|---|---|---|---|
| P500 | 718 | 1.27 | 0.234 | 0.73 | 0.50 | 0.084 |
| P600 | 791 | 1.26 | 0.258 | 1.72 | 1.46 | 0.045 |
| K600 | 806 | 0.91 | 0.263 | 0.33 | 0.06 | 0.245 |
| K700 | 761 | 1.00 | 0.236 | 0.35 | 0.12 | 0.177 |
| K800 | 893 | 1.22 | 0.315 | 0.69 | 0.38 | 0.169 |
| BNCA | XPS (wt%) | EDX (wt%) | Wavelength (cm−1) | ID/IG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | N1s | P2p | O/C (%) | C | O | N | P | D | G | ||
| P500 | 84.7 | 11.7 | 0.5 | 3.1 | 13.8 | 31.2 | 49.6 | - | 19.2 | 1336.5 | 1585.6 | 2.00 |
| P600 | 68.2 | 20.4 | 0.2 | 11.1 | 29.9 | 40.1 | 5.1 | - | 54.7 | 1333.6 | 1585.6 | 2.03 |
| K600 | 81.9 | 17.5 | 0.6 | - | 21.4 | 82.4 | 17.6 | - | - | 1342.3 | 1583.7 | 1.87 |
| K700 | 84.8 | 15.0 | 0.16 | - | 17.7 | 70.0 | 30.0 | - | - | 1345.2 | 1590.5 | 1.86 |
| K800 | 82.9 | 16.88 | 0.21 | - | 20.4 | 67.0 | 33.0 | - | - | 1337.5 | 1590.5 | 1.89 |
| BNCA | CCV (F g−1)/Vb (mV s−1) | CEIS (F g−1) | ESR (Ω) | −φ (°) | τ (s) | IC (μF cm−2) | |||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 25 | ||||||
| P500 | 112 | 84 | 64 | 43 | 99.8 | 23.2 | 68.1 | 159.2 | 13.9 |
| P600 | 107 | 103 | 94 | 78 | 129.9 | 7.4 | 73.3 | 5.8 | 16.4 |
| K600 | 119 | 79 | 57 | 32 | 111.6 | 11.0 | 56.8 | 159.2 | 13.8 |
| K700 | 192 | 182 | 169 | 143 | 181.6 | 8.8 | 78.8 | 2.3 | 23.9 |
| K800 | 180 | 179 | 169 | 151 | 219.5 | 2.9 | 75.7 | 0.9 | 24.6 |
| Reference | Cellulose | Dopant Agent | SBET (m2 g−1) | Capacitance (F g−1) | |
|---|---|---|---|---|---|
| Wang et al. [44] | Hydrolyzed cotton | N: | Urea | 123–366 | 220–275 3E 6 M KOH |
| Li et al. [45] | BC, Hainan Yide Food Industry Co. | N, P, and S: | (NH4)H2PO4, (NH4)2SO4 (NH4)H2PO4/(NH4)2SO4 | 296–498 | 80–255 2 M H2SO4 |
| Liu et al. [46] | BC, Guilin Qi Hong Technology Co. | N, P, and S: | Thiourea (CH4N2S) Rogor (C5H12NO3PS2, 40 wt%) Phoxim (C12H15N2O3PS, 40 wt%) | 261–589 | 132–509 3E and 2E 0.5 M H2SO4 |
| Fang et al. [47] | BC, Hainan Yide Food Industry Co. | O: | Juglone (5-hy-droxy-1,4-naphthalenedione) Carbon nanotubes | 95–102 | 74.8–461.8 3E 1 M H2SO4 |
| Wang et al. [48] | BC, Hainan Yeguo Food Company | N: | Polypyrrole Carbon nanotubes (20–50 µm) | – | 0.15–0.5 F cm−2 Gel PVA/H2SO4 |
| Zhang and Chen [49] | BC, Hainan Yide Food Co. | N: | Polydopamine | 191–464 | 26–260 2E and Coin CR2032 1 M H2SO4 |
| Bai et al. [13] | BC. Yeguo Foods Co. | O: | Poly(ethylene-co-vinyl alcohol) | 747–2189 | 420 3E 6 M KOH |
| Wannasen et al. [50] | BC. Gluconacetobacter xylinum en D-glucose | N, P, Co: | Cobalt(II) nitrate hexahydrate Phosphoric acid | 12–44 | 43.3 (158.5 mF cm−2) 3E 3 M KOH |
| Xu et al. [51] | BC. Hainan Yide Foods Co., Ltd. | N: | 1 M Pyrrole | – | 459.5 3E 1 M NaCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal-Rueda, J.; Zapata-Benabithe, Z.; Posada, L.; Martínez, E.; Herrera, S.; López, S.; Sobrido, A.B.J.; Castro, C.I. Bacterial Nanocellulose from Komagataeibacter Medellinensis in Fique Juice for Activated Carbons Production and Its Application for Supercapacitor Electrodes. Polymers 2023, 15, 1760. https://doi.org/10.3390/polym15071760
Villarreal-Rueda J, Zapata-Benabithe Z, Posada L, Martínez E, Herrera S, López S, Sobrido ABJ, Castro CI. Bacterial Nanocellulose from Komagataeibacter Medellinensis in Fique Juice for Activated Carbons Production and Its Application for Supercapacitor Electrodes. Polymers. 2023; 15(7):1760. https://doi.org/10.3390/polym15071760
Chicago/Turabian StyleVillarreal-Rueda, Juliana, Zulamita Zapata-Benabithe, Laia Posada, Estefanía Martínez, Sara Herrera, Stiven López, Ana B. J. Sobrido, and Cristina I. Castro. 2023. "Bacterial Nanocellulose from Komagataeibacter Medellinensis in Fique Juice for Activated Carbons Production and Its Application for Supercapacitor Electrodes" Polymers 15, no. 7: 1760. https://doi.org/10.3390/polym15071760
APA StyleVillarreal-Rueda, J., Zapata-Benabithe, Z., Posada, L., Martínez, E., Herrera, S., López, S., Sobrido, A. B. J., & Castro, C. I. (2023). Bacterial Nanocellulose from Komagataeibacter Medellinensis in Fique Juice for Activated Carbons Production and Its Application for Supercapacitor Electrodes. Polymers, 15(7), 1760. https://doi.org/10.3390/polym15071760