Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Eudragit E PO-LA Films
2.3. Swelling and Disintegration Time Determination
2.4. Evaluation of the Mechanical Properties of Films
2.5. Evaluation of the Mucoadhesivity
2.6. Differential Scanning Calorimetry Measurements
2.7. Drug Release from Eudragit E PO Films
2.8. Particle Size and Zeta Potential
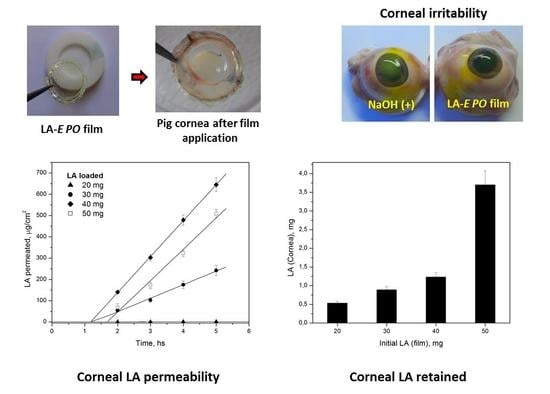
2.9. Corneal Irritability Evaluation
2.10. Corneal Permeability
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of LA on the Mechanical Properties of Eudragit E PO Films
3.2. Film Swelling and Stability
3.3. Mucoadhesivity
3.4. DSC
3.5. LA Release
3.6. Particle Size and Zeta Potential
3.7. Corneal Permeability
3.8. Corneal Irritability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burt Berkson, M.D. The Alpha Lipoic Acid Breakthrough; Prima Publishing: Roseville, CA, USA; New York, NY, USA, 1998. [Google Scholar]
- Biewenga, G.P.; Haenen, G.; Bast, A. Lipoic Acid in Health and Disease. In Book an Overview of Lipoate Chemistry; Fuchs, J., Packer, L., Zimmer, G., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 1–32. [Google Scholar]
- Alvarez-Rivera, F.; Fernandez-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgindy, N.; Samy, W. Evaluation of the mechanical properties and drug release of cross-linked Eudragit films containing metronidazole. Int. J. Pharm. 2009, 376, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eudragit Acrylic Polymers for Pharmaceutical Applications. Röhm Deguss-Hüls Group. Pharma Polymers. Available online: https://www.pharmaceuticalonline.com/doc/rohm-america-0003 (accessed on 9 February 2023).
- Priemel, P.A.; Laitinen, R.; Grohganz, H.; Rades, T.; Strachan, C.J. In situ amorphisation of indomethacin with Eudragit® E during dissolution. Eur. J. Pharm. Biopharm. 2013, 85, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Doreth, M.; Löbmann, K.; Grohganz, H.; Holm, R.; Lopez de Diego, H.; Rades, T.; Priemel, P.A. Glass solution formation in water—In situ amorphization of naproxen and ibuprofen with Eudragit® E PO. J. Drug Deliv. Sci. Technol. 2016, 34, 32–40. [Google Scholar] [CrossRef]
- Pignatello, R.; Bucolo, C.; Ferrara, P.; Maltese, A.; Puleo, A.; Puglisi, G. Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur. J. Pharm. Sci. 2002, 16, 53–61. [Google Scholar] [CrossRef]
- Safwat, S.M.; Al-Kassas, R.S. Evaluation of gentamicin-Eudragit microspheres as ophthalmic delivery systems in inflamed rabbit’s eyes. STP Pharma Sci. 2002, 12, 357–361. [Google Scholar]
- Glaessi, B.; Siepmann, F.; Tucker, I.; Rades, T.; Siepmann, J. Deeper insight into the drug release mechanisms in Eudragit RL-based delivery systems. Int. J. Pharm. 2010, 389, 139–146. [Google Scholar] [CrossRef]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated β-Cyclodextrin-Loaded Eudragit® RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Taghe, S.; Alany, R.G.; Nokhodchi, A. Eudragit® L100/Polyvinyl Alcohol Nanoparticles Impregnated Mucoadhesive Films as Ocular Inserts for Controlled Delivery of Erythromycin: Development, Characterization and In Vivo Evaluation. Biomedicines 2022, 10, 1917. [Google Scholar] [CrossRef]
- Martinez, S.M.; Inda, A.; Garcia, A.M.; Bermúdez, J.M.; Gonzo, E.E.; Herrero-Vanrell, R.; Luna, J.D.; Allemandi, D.A.; Quinteros, D.A. Development of melatonin-loaded, human-serum-albumin nanoparticles formulations using different methods of preparation for ophthalmic administration. Int. J. Pharm. 2022, 628, 122308. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Rathore, K.S.; Nema, R.K. Review on ocular inserts. Int. J. Pharmtech. Res. 2009, 1, 164–169. [Google Scholar]
- Chang, J.N. Recent advances in ophthalmic drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 165–192. [Google Scholar]
- Jain, D.; Carvalho, E.; Banerjee, E. Biodegradable hybrid polymeric membranes for ocular drug delivery. Acta Biomater. 2010, 6, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, D.; Wang, H.; Zou, H.; Bai, T.; Feng, C.; Lin, Q. “Kill-release” antibacterial polysaccharides multilayer coating based therapeutic contact lens for effective bacterial keratitis treatment. RSC Adv. 2021, 11, 26160–26167. [Google Scholar] [CrossRef]
- Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Bertoni, M.; Caramella, C. Characterization of mucin interaction with three viscosity grades of sodium carboxymethylcellulose. Comparison between rheological and tensile testing. Eur. J. Pharm. Sci. 1996, 4, 189–196. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Steele, F.; Khutoryanskiy, V.V. Polyaphron formulations stabilised with different water-soluble polymers for ocular drug delivery. Pharmaceutics 2022, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Test N° 437: Bovine Corneal Opacity and Permeability Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage; OECD Publishing: Paris, France, 2020. [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Meier, M.M.; Kanis, L.A.; Soldi, V. Characterization and drug-permeation profiles of microporous and dense cellulose acetate membranes: Influence of plasticizer and pore forming agent. Int. J. Pharm. 2004, 278, 99–110. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y. A study of release mechanisms of different ophtalmic drugs from erodible ocular inserts based on poly(ethylene oxide). Eur. J. Pharm. Biopharm. 2002, 54, 193–199. [Google Scholar] [CrossRef]
- Hornyak, G.L.; Moore, J.J.; Tibbals, H.F.; Dutta, J. Fundamentals of Nanotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781420048049. [Google Scholar]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Ferraboschi, I.; Delledonne, A.; Pescina, S.; Padula, C.; Santi, P.; Sissa, C.; Terenziani, F.; Nicoli, S. Cyclosporine-loaded micelles for ocular delivery: Investigating the penetration mechanisms. J. Control. Release 2022, 349, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy—An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Harnessing the tunable cavity of nanoceria for enhancing Y-27632-mediated alleviation of ocular hypertension. Theranostics 2021, 11, 5447–5463. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, X.; Zhang, N.; Jiao, X.; Ma, Y.; Liu, R.; Liu, B.; Li, Z. Targeted drug delivery vehicles mediated by nanocarriers and aptamers for posterior eye disease therapeutics: Barriers, recent advances and potential opportunities. Nanotechnology 2022, 33, 162001. [Google Scholar] [CrossRef] [PubMed]
- Eurl Ecvam Status Report. Non-Animal Methods in Science and Regulation; Publications Office of the European Union: Luxembourg, 2021; ISSN 1831-9424. [Google Scholar] [CrossRef]
- Efron, N.; Morgan, P.B.; Katsara, S.S. Validation of grading scales for contact lens complications. Ophthal. Physiol. Opt. 2001, 21, 17–29. [Google Scholar] [CrossRef]








| LA:E PO w/w Ratio | Tensile Strength ± SD (N/cm2) | Young’s Modulus ± SD (N/cm2) | Adhesion Work ± SD (cN.mm) | Film Desintegration Time * |
|---|---|---|---|---|
| 0.0:1 | - | - | 2.69 ± 0.14 | >15 d |
| 0.2:1 | 11.62 ± 1.27 | 3.67 ± 0.23 | 5.73 ± 0.31 | 24 h |
| 0.3:1 | 6.00 ± 0.54 | 1.84 ± 0.10 | 10.47 ± 0.62 | 3 h |
| 0.4:1 | 3.41 ± 0.27 | 0.73 ± 0.03 | 13.15 ± 0.70 | 2 h |
| 0.5:1 | 2.48 ± 0.19 | 0.42 ± 0.02 | 14.24 ± 0.74 | 1.5 h |
| Sample | Transition Temperature (°C) | ΔH (J g−1) |
|---|---|---|
| LA | 64.7 | 125.03 |
| Eudragit E PO | 63.1 | 6.25 |
| E PO:PEG400, 1:0.06 | 48.5 | 3.78 |
| E PO:PEG400:LA, 1:0.06:0.2 | 27.9 | 1.58 |
| E PO:PEG400:LA, 1:0.06:0.3 | 29.3 | 2.38 |
| E PO:PEG400:LA, 1:0.06:0.4 | 27.8 | 1.19 |
| E PO:PEG400:LA, 1:0.06:0.5 | 26.9 | 1.37 |
| LA Loaded, (mg) | n | r2 |
|---|---|---|
| 20 | 0.493 ± 0.015 | 0.997 |
| 30 | 0.828 ± 0.040 | 0.996 |
| 40 | 0.537 ± 0.012 | 0.998 |
| 50 | 0.621 ± 0.018 | 0.997 |
| Sample E PO:PEG400:LA | Time (h) | Particle Size (nm) | Polydispersion Index | Zeta Potential (mV) |
|---|---|---|---|---|
| 1:0.06:0.2 | 0 | 891.5 | 0.74 | −6.9 |
| 0.5 | 593.6 | 0.50 | −3.8 | |
| 1.5 | 725.0 | 0.62 | −0.1 | |
| 3 | 789.6 | 0.77 | +0.8 | |
| 6 | 661.3 | 0.63 | +10.9 | |
| 24 | 78.0 | 0.32 | +15.3 | |
| 1:0.06:0.3 | 0 | 1436.0 | 0.755 | −8.2 |
| 0.5 | 697.5 | 0.483 | −2.8 | |
| 1.5 | 31.4 | 0.317 | +7.9 | |
| 3 | 23.5 | 0.361 | +13.1 | |
| 6 | 20.9 | 0.308 | +11.9 | |
| 24 | 20.1 | 0.381 | +12.6 | |
| 1:0.06:0.4 | 0 | 244.2 | 0.40 | −4.2 |
| 0.5 | 17.2 | 0.45 | +11.4 | |
| 1.5 | 17.4 | 0.42 | +6.9 | |
| 3 | 13.1 | 0.29 | +13.9 | |
| 6 | 24.4 | 0.34 | +13.7 | |
| 24 | 12.3 | 0.28 | +14.5 | |
| 1:0.06:0.5 | 0 | 494 | 0.51 | −15.7 |
| 0.5 | 47.5 | 0.36 | +16.6 | |
| 1.5 | 76.3 | 0.34 | +15.3 | |
| 3 | 37.8 | 0.27 | +16.3 | |
| 6 | 34.2 | 0.25 | +17.2 | |
| 24 | 55.5 | 0.34 | +18.6 | |
| E PO-PEG400/PBS 1:0.06 | 0 | 1230.0 | 0.83 | −9.3 |
| 3 | 3261.0 | 0.86 | −10.3 | |
| 6 | 4271.0 | 0.53 | −10.7 | |
| 24 | 2376.0 | 0.83 | −15.2 | |
| LA (50 mg)/PBS | 0 | 109.8 | 0.35 | −21.8 |
| 3 | 112.8 | 0.62 | −21.3 | |
| 6 | 744.9 | 0.55 | −17.6 | |
| 24 | 309.7 | 0.54 | −21.3 | |
| PBS | - | 19.4 | 0.52 | −16.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierbrauer, K.L.; Comini, L.R.; Leonhard, V.; Escobar Manzanelli, M.A.; Castelli, G.; Farfán, S.; Alasino, R.V.; Beltramo, D.M. Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers 2023, 15, 1793. https://doi.org/10.3390/polym15071793
Bierbrauer KL, Comini LR, Leonhard V, Escobar Manzanelli MA, Castelli G, Farfán S, Alasino RV, Beltramo DM. Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers. 2023; 15(7):1793. https://doi.org/10.3390/polym15071793
Chicago/Turabian StyleBierbrauer, Karina L., Laura R. Comini, Victoria Leonhard, Micaela A. Escobar Manzanelli, Gabriela Castelli, Silvia Farfán, Roxana V. Alasino, and Dante M. Beltramo. 2023. "Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability" Polymers 15, no. 7: 1793. https://doi.org/10.3390/polym15071793
APA StyleBierbrauer, K. L., Comini, L. R., Leonhard, V., Escobar Manzanelli, M. A., Castelli, G., Farfán, S., Alasino, R. V., & Beltramo, D. M. (2023). Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers, 15(7), 1793. https://doi.org/10.3390/polym15071793