Application of Tamarind Shell as a Green Additive in Natural Rubber
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
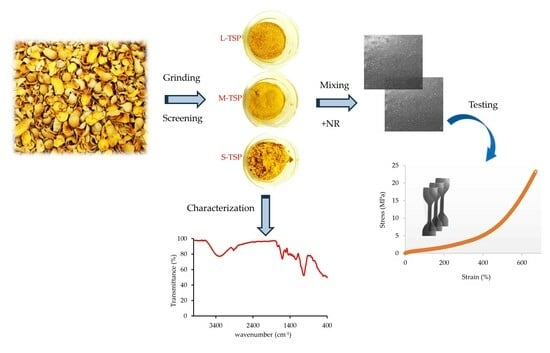
2.2. Preparation and Characterization of Tamarind Shell Powder (TSP)
2.3. Rubber Compound Preparation and Testing
3. Results and Discussion
3.1. Basic Characterization of TSP
3.2. Properties of Rubber Compounds and Vulcanizates
3.2.1. Cure Characteristics
3.2.2. Mechanical and Aging Resistance Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purushotham, G.; Yathin, K.L. Study of Mechanical behavior for tamarind shell powder and coconut coir fiber epoxy composite for aerospace application. Int. J. Trend Sci. Res. Dev. 2018, 3, 941–949. [Google Scholar] [CrossRef]
- Brailson, M.B.; Binoj, J.S.; Prem, S.N.; Hassan, S.A.; Siengchin, S.; Sanjay, M.R.; Liu, Y.C. Sustainable development in utilization of Tamarindus indica L. and its by-products in industries: A review. Curr. Res. Green Sustain. Chem. 2021, 4, 100207. [Google Scholar]
- Kumar, N.S.; Shaikh, H.M.; Asif, M.; Al-Ghurabi, E.H. Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci. Rep. 2021, 11, 2586. [Google Scholar] [CrossRef] [PubMed]
- Wadekar, M.; Rode, C.; Bendale, Y.; Patil, K.; Gaikwad, A.; Prabhune, A. Effect of calcination cycles on the preparation of tin oxide based traditional drug: Studies on its formation and characterization. J. Pharm. Biomed. Anal. 2006, 41, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, I.A.; Oderinde, R.A.; Kajogbola, D.O.; Uponi, J.I. Oil content and fatty acid composition of some underutilized legumes from Nigeria. Food Chem. 2006, 99, 115–120. [Google Scholar] [CrossRef]
- Christian, S.N.; Cecil, K.K.; Thomas, T.K. Tamarindus Indica fruit shell ash: A low cost and effective catalyst for biodiesel production from Parinari curatellifolia seeds oil. SN Appl. Sci. 2019, 1, 253. [Google Scholar]
- Amos, O.; Ogunniyi, D.S.; Odetoye, T.E. Production of biodiesel from Parinari polyandra B. seed oil using bio-based catalysts. J. Technol. Dev. 2016, 13, 26. [Google Scholar] [CrossRef]
- Taiwo, O.; Osinowo, F. Evaluation of various agro-wastes for traditional black soap production. Bioresour. Technol. 2001, 79, 95–97. [Google Scholar] [CrossRef]
- Luengthanaphol, S.; Mongkholkhajornsilp, D.; Douglas, S.; Douglas, P.L.; Pengsopa, L.I.; Pongamphai, S. Extraction of antioxidants from sweet Thai tamarind seed coat––Preliminary experiments. J. Food Eng. 2004, 63, 247–252. [Google Scholar] [CrossRef]
- Sudjaroen, Y.; Haubner, R.; Würtele, G.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Changbumrung, S.; Bartsch, H.; Owen, R.W. Isolation and structure elucidation of phenolic antioxidants from tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005, 43, 1673–1682. [Google Scholar] [CrossRef]
- Abdulmajid, A.; Hamidon, T.S.; Abdul Rahim, A.A.; Hussin, M.H. Physicochemical studies of tamarind shell tannins as a potential green rust converter. BioResources 2019, 14, 6863–6882. [Google Scholar] [CrossRef]
- Thirumal, V.; Dhamodharan, K.; Yuvakkumar, R.; Ravi, G.; Saravanakumar, B.; Thambidurai, M.; Dang, C.; Dhayalan, V. Cleaner production of tamarind fruit shell into bio-mass derived porous 3D-activated carbon nanosheets by CVD technique for supercapacitor applications. Chemosphere 2021, 282, 131033. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Edathil, A.A.; Yasun, Y.; Song, K.J.-K.; Haija, M.A.; Banat, F. Tamarind shell derived N-doped carbon for capacitive deionization (CDI) studies. J. Electroanal. Chem. 2019, 848, 113307. [Google Scholar] [CrossRef]
- Sivasankar, V.S.; Rajkumar, S.; Murugesh, A.D. Tamarind (Tamarindus indica) fruit shell carbon: A calcium-rich promising adsorbent for fluoride removal from groundwater. J. Hazard. Mater. 2012, 225–226, 164–172. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S.; Sanjeeviraja, C. High performance solid-state electric double layer capacitor from redox mediated gel polymer electrolyte and renewable tamarind fruit shell derived porous carbon. J. Hazard. Mater. 2012, 225–226, 164–172. [Google Scholar] [CrossRef]
- Ahmad, K.Z.K.; Harun, A.; Faidzi, M.K.; Othman, R.N.; Kamarolzaman, A.A. Mechanical and thermal properties of epoxy/tamarind shell composite. J. Kejuruter. 2021, SI 4, 87–93. [Google Scholar] [CrossRef]
- Ashok, B.; Hariram, N.; Siengchin, S.; Rajulu, A.V. Modification of tamarind fruit shell powder with in situ generated copper nanoparticles by single step hydrothermal method. J. Bioresour. Bioprod. 2020, 5, 180–185. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Natarajan, H.; Zhang, J.; Ashok, B.; Anumakonda, V.R. Modification of agricultural waste tamarind fruit shell powder by in situ generation of silver nanoparticles for antibacterial filler applications. J. Polym. Anal. Charact. 2019, 24, 421–427. [Google Scholar] [CrossRef]
- Suguna, M.; Kumar, N.S.; Subbaiah, M.V.; Krishnaiah, A. Removal of divalent manganese from aqueous solution using Tamarindus indica fruit nut shell. J. Chem. Pharm. Res. 2010, 2, 7–20. [Google Scholar]
- ISO 6502-3; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Curemeter. ISO: Geneva, Switzerland, 2023.
- ISO 48-4; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 4: Indentation Hardness by Durometermethod (Shore Hardness). ISO: Geneva, Switzerland, 2018.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ISO 4649; Rubber, Vulcanized or Thermoplastic—Determination of Abrasion Resistance Using a Rotating Cylindrical Drum Device. ISO: Geneva, Switzerland, 2017.
- ISO 188; Rubber, Vulcanized or Thermoplastic—Accelerated ageing and heat resistance tests. ISO: Geneva, Switzerland, 2023.
- Li, W.; Huang, R.; Han, S.; Li, X.; Gong, H.; Zhang, Q.; Yan, C.; Li, Y.; He, R. Potential of tamarind shell extract against oxidative stress in vivo and in vitro. Molecules 2023, 28, 1885. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.; Kumar, S.H.A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Klongklaew, P.; Khamjapo, P.; Sae-Oui, P.; Jittham, P.; Loykulnant, S.; Intiya, W. Characterization and application in natural rubber of Leucaena leaf and its extracted products. Polymers 2023, 15, 3698. [Google Scholar] [CrossRef]
- Silva, J.E.; Calixto, G.Q.; de Almeida, C.C.; Melo, D.M.A.; Melo, M.A.F.; Freitas, J.C.O. Energy potential and thermogravimetric study of pyrolysis kinetics of biomass wastes. J. Therm. Anal. Calorim. 2019, 137, 1635–1643. [Google Scholar] [CrossRef]
- Siriwong, C.; Boopasiri, S.; Jantarapibun, V.; Kongsook, B.; Pat-tanawanidchai, S.; Sae-Oui, P. Properties of natural rubberfilled with untreated and treated spent coffee grounds. J. Appl. Polym. Sci. 2017, 135, 46060. [Google Scholar] [CrossRef]
- Ashok, B.; Nanthagopal, K.; Krishnan, R.; Rayapati, S.; Rajagopal, T.K.R.T.K. Lemon peel oil—A novel renewable alternative energy source for diesel engine. Energy Convers. Manag. 2017, 139, 110–121. [Google Scholar] [CrossRef]
- Amulani, A.; Nandanwar, T.; Baskaran, K.; Prakash, R.; Mohan, C.G. Characterization of tamarind biomass to substantiate the feasibility towards alternative fuel. Sustain. Energy Technol. Assess. 2023, 56, 103056. [Google Scholar] [CrossRef]
- Akhtar, N.; Goyal, D.; Goyal, A. Physico-chemical characteristics of leaf litter biomass to delineate the chemistries involved in biofuel production. J. Taiwan Inst. Chem. Eng. 2016, 62, 239–246. [Google Scholar] [CrossRef]
- Tapangnoi, P.; Sae-Oui, P.; Naebpetch, W.; Siriwong, C. Preparation of purified spent coffee ground and its reinforcement in natural rubber composite. Arab. J. Chem. 2022, 15, 103917. [Google Scholar] [CrossRef]
- Boopasiri, S.; Sae-Oui, P.; Lundee, S.; Takaewnoi, S.; Siriwong, C. Reinforcing efficiency of pyrolyzed spent coffee ground in styrene-butadiene rubber. Macromol. Res. 2021, 29, 597–604. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Pin, G.; Vergara-Castan, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acidnanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495. [Google Scholar] [CrossRef]
- Magalhaes, C.N.A.; Iraiton, I.A.; Gomes, M.G.; Simone, S.S.S. Estudos de novas fonts minerais em produtos naturals. Rev. Bras. Farm. 2004, 85, 61–63. [Google Scholar]
- Butler, J.; Freakley, P.K. Effect of humidity and water content on the cure behavior of a natural-rubber accelerated sulfur compound. Rubber Chem. 1992, 65, 374–384. [Google Scholar] [CrossRef]
- Radhakrishnan, P.G.; Varghese, S.P.; Das, B.C. Application of ethylenediamine hydroxypropyl tamarind fruit shell as adsorbent to remove Eriochrome black T from aqueous solutions—Kinetic and equilibrium studies. Separ. Sci. Technol. 2018, 53, 417–438. [Google Scholar] [CrossRef]
- Zhan, Y.-H.; Wei, Y.-C.; Tian, J.-j.; Gao, Y.-Y.; Luo, M.-C.; Liao, S. Effect of protein on the thermogenesis performance of natural rubber matrix. Sci. Rep. 2020, 10, 16417. [Google Scholar] [CrossRef]
- Lhamo, D.; McMahan, C. Effect of protein addition on properties of guayule natural rubber. Rubber Chem. Technol. 2017, 90, 387–404. [Google Scholar] [CrossRef]
- Liu, X.-X.; He, M.-F.; Luo, M.-C.; Wei, Y.-C.; Liao, S. The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks. E-Polymer 2022, 22, 445–453. [Google Scholar] [CrossRef]
- Smitthipong, W.; Tantatherdtam, R.; Rungsanthien, K.; Suwanruji, P.; Klanarong, S.; Radabutra, S.; Thanawan, S.; Vallat, M.F.; Nardin, M.; Mougin, K.; et al. Effect of non-rubber components on properties of sulphur crosslinked natural rubbers. Adv. Mater. Res. 2014, 844, 345–348. [Google Scholar] [CrossRef]
- Wei, Y.C.; Liu, G.X.; Zhang, L.; Zhao, F. Exploring the unique characteristics of natural rubber induced by coordination interaction between proteins and Zn2+. Polymer 2022, 193, 122357. [Google Scholar] [CrossRef]
- Sareena, C.; Ramesan, M.T.; Purushothaman, E. Utilization of peanut shell powder as a novel filler in natural rubber. J. Appl. Polym. Sci. 2012, 125, 2322–2334. [Google Scholar] [CrossRef]









| Ingredient | Function | Supplier |
|---|---|---|
| Carbon black (N330) | Reinforcing filler | Thai Carbon Black PCL. (Angthong, Thailand) |
| Zinc oxide (ZnO, white seal) | Inorganic activator | Thai-Lysaght Co., Ltd. (Ayutthaya, Thailand) |
| Stearic acid | Organic activator | Kij Paiboon Chemical LP. (Bangkok, Thailand) |
| N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) | Antidegradant | Reliance Co., Ltd. (Bangkok, Thailand) |
| Aromatic oil | Plasticizer | Union Link Co., Ltd. (Samutprakan, Thailand) |
| N-tert-butylbenzothiazole-2-sulfenamide (TBBS) | Accelerator | Monflex Pte. Ltd. (Singapore) |
| Sulfur | Vulcanizing agent | Siam Chemical Industry Co., Ltd. (Samutprakan, Thailand) |
| Ingredient | Content (Parts per Hundred Rubber: phr) |
|---|---|
| Natural rubber (STR 5 L) | 100 |
| Zinc oxide | 3 |
| Stearic acid | 1 |
| TSP * | 0, 2, 4, 6, 8, and 10 |
| Carbon black (N330) | 40 |
| 6PPD | 1 |
| Aromatic oil | 2 |
| TBBS | 1 |
| Sulfur | 2 |
| Sample | Average Particle Size (µm) | Moisture Content (%) | Density (g/cm3) | pH |
|---|---|---|---|---|
| L-TSP | 191.5 ± 0.1 | 5.7 ± 0.3 | 1.56 ± 0.04 | 4.5 ± 0.1 |
| M-TSP | 29.8 ± 0.1 | 6.7 ± 0.4 | ||
| S-TSP | 17.2 ± 0.0 | 6.7 ± 0.3 |
| Compound | TSP Content (phr) | Scorch Time, ts1, (min) | Optimum Cure Time, tc95, (min) | Torque Difference, MH − ML, (dN.m) |
|---|---|---|---|---|
| NR/Control | 0 | 2.6 | 7.8 | 13.9 |
| NR/L-TSP | 2 | 2.5 | 7.6 | 13.6 |
| 4 | 2.5 | 7.4 | 13.6 | |
| 6 | 2.4 | 7.2 | 13.6 | |
| 8 | 2.2 | 7.0 | 13.6 | |
| 10 | 2.3 | 7.2 | 13.7 | |
| NR/M-TSP | 2 | 2.4 | 7.3 | 13.3 |
| 4 | 2.2 | 7.3 | 12.9 | |
| 6 | 2.1 | 7.0 | 12.4 | |
| 8 | 1.9 | 7.1 | 12.7 | |
| 10 | 1.7 | 6.0 | 11.6 | |
| NR/S-TSP | 2 | 2.4 | 7.2 | 12.8 |
| 4 | 2.1 | 7.2 | 12.9 | |
| 6 | 2.1 | 7.2 | 12.3 | |
| 8 | 2.0 | 7.3 | 12.2 | |
| 10 | 1.9 | 7.2 | 11.7 |
| Compound | Content (phr) | Hardness (Shore A) | Tensile Strength (MPa) | Stress at 100 Elongation (MPa) | Elongation at Break (%) | Volume Loss (mm3) |
|---|---|---|---|---|---|---|
| NR/Control | 0 | 61.6 ± 0.2 | 28.5 ± 0.9 | 3.13 ± 0.13 | 487 ± 11 | 124.7 ± 0.5 |
| NR/L-TSP | 2 | 61.9 ± 0.2 | 25.9 ± 0.4 | 3.17 ± 0.14 | 430 ± 14 | 119.8 ± 2.2 |
| 4 | 62.1 ± 0.4 | 24.0 ± 0.3 | 3.22 ± 0.19 | 409 ± 12 | 125.2 ± 1.8 | |
| 6 | 63.2 ± 0.3 | 20.1 ± 0.4 | 3.27 ± 0.15 | 393 ± 13 | 129.8 ± 0.8 | |
| 8 | 62.5 ± 0.3 | 20.9 ± 0.2 | 3.27 ± 0.16 | 374 ± 15 | 131.7 ± 0.7 | |
| 10 | 63.5 ± 0.2 | 19.1 ± 0.1 | 3.30 ± 0.22 | 372 ± 8 | 135.4 ± 0.7 | |
| NR/M-TSP | 2 | 62.4 ± 0.5 | 26.3 ± 0.4 | 3.23 ± 0.20 | 437 ± 11 | 126.4 ± 3.4 |
| 4 | 62.4 ± 0.4 | 26.3 ± 0.7 | 3.19 ± 0.07 | 429 ± 11 | 129.5 ± 2.6 | |
| 6 | 62.9 ± 0.2 | 25.2 ± 0.4 | 3.24 ± 0.13 | 434 ± 7 | 133.7 ± 1.0 | |
| 8 | 63.0 ± 0.3 | 24.1 ± 0.2 | 3.25 ± 0.24 | 427 ± 16 | 138.4 ± 2.1 | |
| 10 | 62.8 ± 0.2 | 22.8 ± 0.3 | 3.29 ± 0.19 | 417 ± 16 | 145.7 ± 2.8 | |
| NR/S-TSP | 2 | 62.4 ± 0.3 | 27.8 ± 1.2 | 3.22 ± 0.04 | 447 ± 15 | 128.3 ± 1.6 |
| 4 | 62.5 ± 0.2 | 27.3 ± 0.1 | 3.23 ± 0.09 | 461 ± 10 | 128.5 ± 4.4 | |
| 6 | 62.8 ± 0.2 | 25.8 ± 0.7 | 3.26 ± 0.11 | 438 ± 20 | 134.6 ± 2.1 | |
| 8 | 63.1 ± 0.2 | 24.7 ± 0.2 | 3.28 ± 0.08 | 426 ± 16 | 140.7 ± 2.6 | |
| 10 | 62.3 ± 0.2 | 23.9 ± 0.3 | 3.38 ± 0.25 | 397 ± 6 | 145.5 ± 4.0 |
| Compound | Content (phr) | Property after the Aging Test | Property Change | ||||
|---|---|---|---|---|---|---|---|
| Hardness (Shore A) | Tensile Strength (MPa) | Elongation at Break (%) | Hardness (Shore A) | Tensile Strength (%) | Elongation at Break (%) | ||
| NR/Control | 0 | 64.9 ± 0.4 | 27.1 ± 0.4 | 448 ± 14 | 3.3 | −4.9 | −8.0 |
| NR/L-TSP | 2 | 65.6 ± 0.2 | 23.5 ± 0.6 | 408 ± 4 | 3.7 | −9.3 | −5.1 |
| 4 | 66.1 ± 0.2 | 22.8 ± 0.4 | 395 ± 14 | 4.0 | −5.0 | −3.4 | |
| 6 | 66.2 ± 0.4 | 20.2 ± 0.4 | 356 ± 14 | 3.0 | 0.5 | −9.4 | |
| 8 | 66.5 ± 0.4 | 20.2 ± 0.6 | 354 ± 13 | 4.0 | −3.3 | −5.3 | |
| 10 | 66.9 ± 0.2 | 18.5 ± 0.4 | 328 ± 8 | 3.4 | −3.1 | −11.8 | |
| NR/M-TSP | 2 | 65.2 ± 0.3 | 26.8 ± 0.8 | 433 ± 11 | 2.8 | 1.9 | −0.9 |
| 4 | 65.4 ± 0.2 | 25.9 ± 0.6 | 414 ± 15 | 3.0 | −1.5 | −3.5 | |
| 6 | 65.7 ± 0.3 | 24.8 ± 0.2 | 410 ± 9 | 2.8 | −1.6 | −5.5 | |
| 8 | 66.5 ± 0.3 | 23.8 ± 0.2 | 409 ± 9 | 3.5 | −1.2 | −4.2 | |
| 10 | 66.0 ± 0.3 | 23.2 ± 0.2 | 409 ± 15 | 3.2 | 1.8 | −1.9 | |
| NR/S-TSP | 2 | 65.8 ± 0.4 | 27.6 ± 0.5 | 438 ± 15 | 3.4 | −0.7 | −2.0 |
| 4 | 65.9 ± 0.3 | 26.5 ± 0.4 | 404 ± 15 | 3.4 | −2.9 | −12.4 | |
| 6 | 65.9 ± 0.3 | 25.9 ± 0.5 | 400 ± 20 | 3.1 | 0.4 | −8.7 | |
| 8 | 66.2 ± 0.2 | 24.2 ± 0.6 | 372 ± 3 | 3.1 | −2.0 | −12.7 | |
| 10 | 66.3 ± 0.4 | 23.9 ± 0.2 | 349 ± 6 | 4.0 | 0.0 | −12.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intiya, W.; Hatthapanit, K.; Thaptong, P.; Sae-oui, P. Application of Tamarind Shell as a Green Additive in Natural Rubber. Polymers 2024, 16, 493. https://doi.org/10.3390/polym16040493
Intiya W, Hatthapanit K, Thaptong P, Sae-oui P. Application of Tamarind Shell as a Green Additive in Natural Rubber. Polymers. 2024; 16(4):493. https://doi.org/10.3390/polym16040493
Chicago/Turabian StyleIntiya, Weenusarin, Kannika Hatthapanit, Puchong Thaptong, and Pongdhorn Sae-oui. 2024. "Application of Tamarind Shell as a Green Additive in Natural Rubber" Polymers 16, no. 4: 493. https://doi.org/10.3390/polym16040493
APA StyleIntiya, W., Hatthapanit, K., Thaptong, P., & Sae-oui, P. (2024). Application of Tamarind Shell as a Green Additive in Natural Rubber. Polymers, 16(4), 493. https://doi.org/10.3390/polym16040493