Colorimetric Indicator Based on Gold Nanoparticles and Sodium Alginate for Monitoring Fish Spoilage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
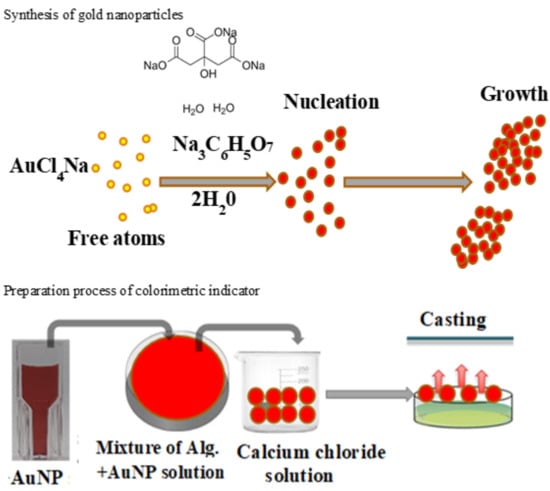
2.2. Synthesis of Gold Nanoparticles (AuNP)
2.3. Colorimetric Indicator Preparation
2.4. Characterization
2.5. Detection Capacity and Colorimetric Analysis
2.6. Evaluation of Indicators under Simulated Conditions (In Vitro)
2.7. Evaluation of the Indicator in Real Packaging Conditions
2.8. Characterization of Fish Samples during Storage
2.9. Determination of Total Volatile Basic Nitrogen (TVB-N)
2.10. Microbiological Analysis
2.11. Statistical Analysis
3. Results
3.1. Characterization of Gold Nanoparticles (AuNPs)
3.2. The Development and Characterization of Alg. Bead-Shaped Indicator Film
3.3. Determination of Total Volatile Basic Nitrogen (TVB-N)
3.4. Mesophilic Aerobic Count
3.5. Determination of pH
3.6. Indicator Color Measurement
3.7. Antibacterial Activity of AuNP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pataca, J.K.; Porto-Figueira, P.; Pereira, J.A.; Caldeira, H.; Câmara, J.S. Profiling the occurrence of biogenic amines in different types of tuna samples using an improved analytical approach. LWT 2020, 139, 110804. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An overview of histamine and other biogenic amines in fish and fish products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zang, J.; Regenstein, J.M.; Xia, W. Technological roles of microorganisms in fish fermentation: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.D.C. Histamine and other biogenic amines in food. From scombroid poisoning to histamine intolerance. Biog. Amines 2019, 1. [Google Scholar] [CrossRef]
- Fusek, M.; Michálek, J.; Buňková, L.; Buňka, F. Modelling biogenic amines in fish meat in Central Europe using censored distributions. Chemosphere 2020, 251, 126390. [Google Scholar] [CrossRef] [PubMed]
- Senapati, M.; Sahu, P.P. Onsite fish quality monitoring using ultra-sensitive patch electrode capacitive sensor at room temperature. Biosens. Bioelectron. 2020, 168, 112570. [Google Scholar] [CrossRef] [PubMed]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Majdinasab, M.; Hosseini, S.M.H.; Sepidname, M.; Negahdarifar, M.; Li, P. Development of a novel colorimetric sensor based on alginate beads for monitoring rainbow trout spoilage. J. Food Sci. Technol. 2018, 55, 1695–1704. [Google Scholar] [CrossRef]
- Lin, T.; Wu, Y.; Li, Z.; Song, Z.; Guo, L.; Fu, F. Visual monitoring of food spoilage based on hydrolysis-induced silver metallization of au nanorods. Anal. Chem. 2016, 88, 11022–11027. [Google Scholar] [CrossRef]
- Taherkhani, E.; Moradi, M.; Tajik, H.; Molaei, R.; Ezati, P. Preparation of on-package halochromic freshness/spoilage nanocellulose label for the visual shelf life estimation of meat. Int. J. Biol. Macromol. 2020, 164, 2632–2640. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Cao, C. Technologies and fabrication of intelligent packaging for perishable products. Appl. Sci. 2019, 9, 4858. [Google Scholar] [CrossRef]
- Ding, L.; Li, X.; Hu, L.; Zhang, Y.; Jiang, Y.; Mao, Z.; Xu, H.; Wang, B.; Feng, X.; Sui, X. A naked-eye detection polyvinyl alcohol/cellulose-based pH sensor for intelligent packaging. Carbohydr. Polym. 2020, 233, 115859. [Google Scholar] [CrossRef]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- El-Nour, K.M.A.; Salam, E.T.A.; Soliman, H.M.; Orabi, A.S. Gold Nanoparticles as a Direct and Rapid Sensor for Sensitive Analytical Detection of Biogenic Amines. Nanoscale Res. Lett. 2017, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Zhai, X.D.; Shi, J.Y.; Zou, X.B.; Wang, S.; Jiang, C.P.; Zhang, J.J.; Huang, X.W.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization and biological applications. J. Clust. Sci. 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Jadhav, N.R.; Naikwadi, H.S.; Savekar, P.L.; Sapkal, I.D.; Kambli, M.M.; Desai, I.A. Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano 2022, 8, 100076. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar] [CrossRef]
- Kolb, A.N.D.; Johnston, J.H. Colour tuneable anisotropic, water-dispersible gold nanoparticles stabilized by chitosan. Gold Bull. 2016, 49, 1–7. [Google Scholar] [CrossRef]
- Mahatnirunkul, T.; Tomlinson, D.C.; McPherson, M.J.; Millner, P.A. One-step gold nanoparticle size-shift assay using synthetic binding proteins and dynamic light scattering. Sens. Actuators B Chem. 2022, 361, 131709. [Google Scholar] [CrossRef]
- Lin, J.-H.; Tseng, W.-L. Ultrasensitive detection of target analyte-induced aggregation of gold nanoparticles using laser-induced nanoparticle Rayleigh scattering. Talanta 2015, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Moudgil, L.; Singh, B.; Kaushal, A.; Mittal, A.; Saini, G.S.S.; Tripathi, S.K.; Singh, G.; Kaura, A. Understanding the mechanism of replacement of citrate from the surface of gold nanoparticles by amino acids: A theoretical and experimental investigation and their biological application. RSC Adv. 2016, 6, 17373–17383. [Google Scholar] [CrossRef]
- Ranjan, R.; Kirillova, M.A.; Kratasyuk, V.A. Ethylene diamine functionalized citrate-capped gold nanoparticles for metal-enhanced bioluminescence. J. Sib. Fed. Univ. Biol. 2020, 13, 322–330. [Google Scholar] [CrossRef]
- Contreras-Trigo, B.; Díaz-García, V.; Guzmán-Gutierrez, E.; Sanhueza, I.; Coelho, P.; Godoy, S.E.; Torres, S.; Oyarzún, P. Slight ph fluctuations in the gold nanoparticle synthesis process influence the performance of the citrate reduction method. Sensors 2018, 18, 2246. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, P.; Nagahiro, T.; Fukada, N.; Kimura, Y.; Niwano, M.; Tamada, K. Characterization of citrates on gold and silver nanoparticles. J. Colloid Interface Sci. 2015, 438, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Khalkho, B.R.; Deb, M.K.; Kurrey, R.; Sahu, B.; Saha, A.; Patle, T.K.; Chauhan, R.; Shrivas, K. Citrate functionalized gold nanoparticles assisted micro extraction of L-cysteine in milk and water samples using Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120523. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.S.; Dempsey, M.; Whitehead, D.E. The response of citrate functionalised gold and silver nanoparticles to the addition of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 15–24. [Google Scholar] [CrossRef]
- Elbeyli, I.Y. Production of crystalline boric acid and sodium citrate from borax decahydrate. Hydrometallurgy 2015, 158, 19–26. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Hao, H. Investigations on dehydration processes of trisodium citrate hydrates. Front. Chem. Sci. Eng. 2012, 6, 276–281. [Google Scholar] [CrossRef]
- Rajakumar, G.; Gomathi, T.; Rahuman, A.A.; Thiruvengadam, M.; Mydhili, G.; Kim, S.-H.; Lee, T.-J.; Chung, I.-M. Biosynthesis and biomedical applications of gold nanoparticles using eclipta prostrata leaf extract. Appl. Sci. 2016, 6, 222. [Google Scholar] [CrossRef]
- Sadeghi, B.; Mohammadzadeh, M.; Babakhani, B. Green synthesis of gold nanoparticles using Stevia rebaudiana leaf extracts: Characterization and their stability. J. Photochem. Photobiol. B Biol. 2015, 148, 101–106. [Google Scholar] [CrossRef]
- Reddy, G.B.; Ramakrishna, D.; Madhusudhan, A.; Ayodhya, D.; Venkatesham, M.; Veerabhadram, G. Catalytic Reduction of p-Nitrophenol and Hexacyanoferrate (III) by Borohydride Using Green Synthesized Gold Nanoparticles. J. Chin. Chem. Soc. 2015, 62, 420–428. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 1–8. [Google Scholar]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M. Gummy gold and silver nanoparticles of apricot (Prunus armeniaca) confer high stability and biological activity. Arab. J. Chem. 2019, 12, 3977–3992. [Google Scholar] [CrossRef]
- Ho, T.C.; Kim, M.H.; Cho, Y.J.; Park, J.S.; Nam, S.Y.; Chun, B.S. Gelatin-sodium alginate-based films with Pseuderanthemum palatiferum (Nees) Radlk. freeze-dried powder obtained by subcritical water extraction. Food Packag. Shelf Life 2020, 24, 100469. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Monteiro, J.P.; Nocchi, S.R.; da Silva, C.T.P.; Nakamura, C.V.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Preparation and cytotoxicity of N,N,N-trimethyl chitosan/alginate beads containing gold nanoparticles. Int. J. Biol. Macromol. 2015, 72, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mubarak, N.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S.; et al. A critical review on the synthesis of natural sodium alginate based composite materials: An innovative biological polymer for biomedical delivery applications. Processes 2021, 9, 137. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Veisi, H.; Aliani, M.H.; Mohammadi, P.; Karmakar, B. Alginate modified magnetic nanoparticles to immobilization of gold nanoparticles as an efficient magnetic nanocatalyst for reduction of 4-nitrophenol in water. J. Mol. Liq. 2021, 327, 114868. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, M.; Essifi, K.; Elbachiri, A.; Tahani, A. Adsorption of sodium alginate onto sodium montmorillonite. Mater. Today Proc. 2021, 45, 7789–7793. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Khoshnoudi-Nia, S.; Azimifar, Z.; Kamyab, S. Evaluation of the total volatile basic nitrogen (TVB-N) content in fish fillets using hyperspectral imaging coupled with deep learning neural network and meta-analysis. Sci. Rep. 2021, 11, 5094. [Google Scholar] [CrossRef]
- Rizo, A.; Mañes, V.; Fuentes, A.; Fernández-Segovia, I.; Barat, J.M. Physicochemical and microbial changes during storage of smoke-flavoured salmon obtained by a new method. Food Control 2015, 56, 195–201. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, W.; Fan, H.; Xie, J.; Qian, Y.-F. Study on the mobility of water and its correlation with the spoilage process of salmon (Salmo solar) stored at 0 and 4 °C by low-field nuclear magnetic resonance (LF NMR 1H). J. Food Sci. Technol. 2018, 55, 173–182. [Google Scholar] [CrossRef]
- Jia, Z.; Shi, C.; Wang, Y.; Yang, X.; Zhang, J.; Ji, Z. Nondestructive determination of salmon fillet freshness during storage at different temperatures by electronic nose system combined with radial basis function neural networks. Int. J. Food Sci. Technol. 2020, 55, 2080–2091. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Z.; Yang, S.-P.; Cheng, Y.; Qian, Y.-F. Study on the spoilage potential of Pseudomonas fluorescens on salmon stored at different temperatures. J. Food Sci. Technol. 2018, 55, 217–225. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Volatile chemical spoilage indexes of raw Atlantic salmon (Salmo salar) stored under aerobic condition in relation to microbiological and sensory shelf lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W. Recent Applications of Spectroscopic and Hyperspectral Imaging Techniques with Chemometric Analysis for Rapid Inspection of Microbial Spoilage in Muscle Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 478–490. [Google Scholar] [CrossRef]
- Hsiao, H.-I.; Chang, J.-N. Developing a microbial time–temperature indicator to monitor total volatile basic nitrogen change in chilled vacuum-packed grouper fillets. J. Food Process. Preserv. 2017, 41, e13158. [Google Scholar] [CrossRef]
- Wiernasz, N.; Cornet, J.; Cardinal, M.; Pilet, M.-F.; Passerini, D.; Leroi, F. Lactic acid bacteria selection for biopreservation as a part of hurdle technology approach applied on seafood. Front. Mar. Sci. 2017, 4, 119. [Google Scholar] [CrossRef]
- Abel, N.; Rotabakk, B.T.; Rustad, T.; Ahlsen, V.B.; Lerfall, J. Physiochemical and Microbiological Quality of Lightly Processed Salmon (Salmo salar L.) Stored Under Modified Atmosphere. J. Food Sci. 2019, 84, 3364–3372. [Google Scholar] [CrossRef]
- Suchak, N.M.; Desai, P.H.; Deshpande, M.P.; Chaki, S.H.; Pandya, S.J.; Kunjadiya, A.; Bhatt, S.V. Study on the concentration of gold nanoparticles for antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035003. [Google Scholar] [CrossRef]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dasari, T.P.S.; Deng, H.; Yu, H. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Heath Part C 2015, 33, 286–327. [Google Scholar] [CrossRef] [PubMed]

















| Color Parameters | AuNP | AuNP + ETD |
|---|---|---|
| L* | 10.56 ± 0.48 | 9.96 ± 0.40 |
| a* | 24.47 ± 0.29 | 3.17 ± 0.24 |
| b* | 13.32 ± 0.45 | −6.06 ± 0.48 |
| ΔE | – | 28.81 |
| Samples | Temperature (°C) | T-Onset (°C) | T50 (°C) | T Endset (°C) | Weight Lost (%) |
|---|---|---|---|---|---|
| Sodium Alginate (Alg.) | 30–800 | 49.4 | 89.3 | 168.8 | 23.4 ± 1.3 a |
| 172.8 | 202.3 | 243.7 | 15.8 ± 1.6 a | ||
| 246.5 | 275.4 | 316.7 | 11.1 ± 0.4 a | ||
| 388.3 | 442.6 | 460.3 | 4.4 ± 0.5a | ||
| Indicator (Alg. + AuNP) | 30–800 | 47.8 | 89.9 | 153.9 | 22.2 ± 4 a |
| 172.1 | 218.6 | 237.4 | 9.4 ± 0,7 a | ||
| 239.1 | 291.08 | 314.4 | 12 ± 1.6 a | ||
| 347.8 | 418.2 | 397.2 | 1.7 ± 0.3 a | ||
| 527.8 | 574.4 | 620.8 | 1.7 ± 0.08 a | ||
| Indicator + ETD | 30–800 | 49.5 | 81.8 | 141.2 | 19.9 ± 4 a |
| 207.1 | 235.1 | 259.3 | 19 ± 4.6 a | ||
| 267.3 | 291.2 | 363.1 | 11.3 ± 0.4 a | ||
| 375.2 | 418.6 | 445.5 | 3.6 ± 0.6 a | ||
| 637.6 | 682.4 | 729.8 | 1.9 ± 0.3 a |
| Storage Times (Days) | L* | a* | b* | ΔE |
|---|---|---|---|---|
| 0 | 17.86 ± 3.57 | 33.47 ± 4.09 | 13.01±3.94 | 0 |
| 2 | 9.60 ± 0.32 | 12.42 ± 2.09 | 0.8 0± 0.75 | 25.7 |
| 5 | 24.57 ± 0.87 | 12.18 ± 1.76 | −8.05 ± 0.53 | 30.6 |
| 7 | 10.26 ± 0.12 | 13.39 ± 2.32 | −19.11 ± 0.72 | 38.7 |
| 9 | 8.86 ± 0.61 | 13.73 ± 1.04 | −24.11 ± 1.01 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre, L.; Bruna Bugueño, J.E.; Leyton Bongiorno, P.A.; Torres Mediano, A.; Rodríguez-Mercado, F.J. Colorimetric Indicator Based on Gold Nanoparticles and Sodium Alginate for Monitoring Fish Spoilage. Polymers 2024, 16, 829. https://doi.org/10.3390/polym16060829
Pierre L, Bruna Bugueño JE, Leyton Bongiorno PA, Torres Mediano A, Rodríguez-Mercado FJ. Colorimetric Indicator Based on Gold Nanoparticles and Sodium Alginate for Monitoring Fish Spoilage. Polymers. 2024; 16(6):829. https://doi.org/10.3390/polym16060829
Chicago/Turabian StylePierre, Lissage, Julio Elías Bruna Bugueño, Patricio Alejandro Leyton Bongiorno, Alejandra Torres Mediano, and Francisco Javier Rodríguez-Mercado. 2024. "Colorimetric Indicator Based on Gold Nanoparticles and Sodium Alginate for Monitoring Fish Spoilage" Polymers 16, no. 6: 829. https://doi.org/10.3390/polym16060829
APA StylePierre, L., Bruna Bugueño, J. E., Leyton Bongiorno, P. A., Torres Mediano, A., & Rodríguez-Mercado, F. J. (2024). Colorimetric Indicator Based on Gold Nanoparticles and Sodium Alginate for Monitoring Fish Spoilage. Polymers, 16(6), 829. https://doi.org/10.3390/polym16060829