Strategies for Improving Sustainability in the Development of High-Performance Styrenic Block Copolymers by Developing Blends with Cellulose Derivatives
Abstract
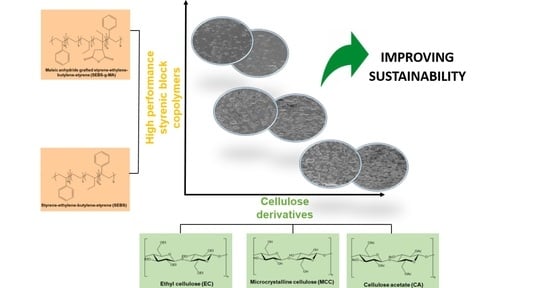
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
3.1. Morphological and Chemical and Surface Properties
3.2. Mechanical Properties
3.3. Thermal Properties
3.4. Electrical Properties
3.5. Indirect Cytotoxicity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Leon, A.C.C.; da Silva, Í.G.M.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High Performance Polymers for Oil and Gas Applications. React. Funct. Polym. 2021, 162, 104878. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Özcan, M. High-Performance Polymers and Their Potential Application as Medical and Oral Implant Materials: A Review. Implant Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-Processable Triarylamine-Based High-Performance Polymers for Resistive Switching Memory Devices. Polym. J. 2016, 48, 117–138. [Google Scholar] [CrossRef]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale Morphology of High-Performance Polymer Solar Cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Chang, L. Recent Advances in High Performance Polymers-Tribological Aspects. Lubricants 2018, 7, 2. [Google Scholar] [CrossRef]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High Performance Polymer Nanocomposites for Additive Manufacturing Applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- DeMeuse, M.T. Polymer Blends Handbook; Utracki, L., Wilkie, C., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Weyhrich, C.W.; Long, T.E. Additive Manufacturing of High-Performance Engineering Polymers: Present and Future. Polym. Int. 2022, 71, 532–536. [Google Scholar] [CrossRef]
- Boydston, A.J.; Cui, J.; Lee, C.U.; Lynde, B.E.; Schilling, C.A. 100th Anniversary of Macromolecular Science Viewpoint: Integrating Chemistry and Engineering to Enable Additive Manufacturing with High-Performance Polymers. ACS Macro Lett. 2020, 9, 1119–1129. [Google Scholar] [CrossRef]
- Yevgen Mamunya, M.I. Advances in Progressive Thermoplastic and Thermosetting Polymers, Perspectives and Applications; Technopress Editura: Lasi, Romania, 2012. [Google Scholar]
- Spontak, R.J.; Patel, N.P. Thermoplastic Elastomers: Fundamentals and Applications. Curr. Opin. Colloid Interface Sci. 2000, 5, 333–340. [Google Scholar] [CrossRef]
- Maji, P.; Naskar, K. Styrenic Block Copolymer-Based Thermoplastic Elastomers in Smart Applications: Advances in Synthesis, Microstructure, and Structure–property Relationships—A Review. J. Appl. Polym. Sci. 2022, 139, 5–7. [Google Scholar] [CrossRef]
- Lin, F.; Wu, C.; Cui, D. Synthesis and Characterization of Crystalline Styrene-b-(Ethylene-Co-Butylene)-b-Styrene Triblock Copolymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1243–1249. [Google Scholar] [CrossRef]
- Downey, A.; Pisello, A.L.; Fortunati, E.; Fabiani, C.; Luzi, F.; Torre, L.; Ubertini, F.; Laflamme, S. Durability and Weatherability of a Styrene-Ethylene-Butylene-Styrene (SEBS) Block Copolymer-Based Sensing Skin for Civil Infrastructure Applications. Sens. Actuators A Phys. 2019, 293, 269–280. [Google Scholar] [CrossRef]
- Wu, T.; Hu, Y.; Rong, H.; Wang, C. SEBS-Based Composite Phase Change Material with Thermal Shape Memory for Thermal Management Applications. Energy 2021, 221, 119900. [Google Scholar] [CrossRef]
- Castro, H.F.; Correia, V.; Pereira, N.; Costab, P.; Oliveiraa, J.; Lanceros-Méndez, S. Printed Wheatstone Bridge with Embedded Polymer Based Piezoresistive Sensors for Strain Sensing Applications. Addit. Manuf. 2018, 20, 119–125. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dai, X.R.; Zou, L.; Wen, S.B.; Sinha, T.K.; Li, H. A Developed, Eco-Friendly, and Flexible Thermoplastic Elastomeric Foam from Sebs for Footwear Application. Express Polym. Lett. 2019, 13, 948–958. [Google Scholar] [CrossRef]
- Chang, Y.W.; Shin, J.Y.; Ryu, S.H. Preparation and Properties of Styrene-Ethylene/Butylene-Styrene (SEBS)-Clay Hybrids. Polym. Int. 2004, 53, 1047–1051. [Google Scholar] [CrossRef]
- Grigorescu, R.M.; Ciuprina, F.; Ghioca, P.; Ghiurea, M.; Iancu, L.; Spurcaciu, B.; Panaitescu, D.M. Mechanical and Dielectric Properties of SEBS Modified by Graphite Inclusion and Composite Interface. J. Phys. Chem. Solids 2016, 89, 97–106. [Google Scholar] [CrossRef]
- Kuester, S.; Merlini, C.; Barra, G.M.O.; Ferreira, J.C.; Lucas, A.; De Souza, A.C.; Soares, B.G. Processing and Characterization of Conductive Composites Based on Poly(Styrene-b-Ethylene-Ran-Butylene-b-Styrene) (SEBS) and Carbon Additives: A Comparative Study of Expanded Graphite and Carbon Black. Compos. Part B Eng. 2016, 84, 236–247. [Google Scholar] [CrossRef]
- Gao, W.; Yu, B.; Li, S.; Chen, S.; Zhu, Y.; Zhang, B.; Zhang, Y.; Cai, H.; Han, B. Preparation and Properties of Reinforced SEBS-Based Thermoplastic Elastomers Modified by PA6. Polym. Eng. Sci. 2022, 62, 1052–1060. [Google Scholar] [CrossRef]
- Yin, L.; Yin, J.; Shi, D.; Luan, S. Effects of SEBS-g-BTAI on the Morphology, Structure and Mechanical Properties of PA6/SEBS Blends. Eur. Polym. J. 2009, 45, 1554–1560. [Google Scholar] [CrossRef]
- Sharudin, R.W.B.; Ohshima, M. Preparation of Microcellular Thermoplastic Elastomer Foams from Polystyrene-b-Ethylene-Butylene-b-Polystyrene (SEBS) and Their Blends with Polystyrene. J. Appl. Polym. Sci. 2013, 128, 2245–2254. [Google Scholar] [CrossRef]
- Abis, L.; Abbondanza, L.; Braglia, R.; Castellani, L.; Giannotta, G.; Po, R. Syndiotactic Polystyrene/High-Density Polyethylene Blends Compatibilized with SEBS Copolymer: Thermal, Morphological, Tensile, Dynamic-Mechanical, and Ultrasonic Characterization. Macromol. Chem. Phys. 2000, 201, 1732–1741. [Google Scholar] [CrossRef]
- Roeder, J.; Oliveira, R.V.B.; Gonçalves, M.C.; Soldi, V.; Pires, A.T.N. Polypropylene/Polyamide-6 Blends: Influence of Compatibilizing Agent on Interface Domains. Polym. Test. 2002, 21, 815–821. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Maji, P.K. Cellulose Nanofibres as Biomaterial for Nano-Reinforcement of Poly[Styrene-(Ethylene-Co-Butylene)-Styrene] Triblock Copolymer. Cellulose 2018, 25, 449–461. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Evtuguin, D.; Barros-Timmons, A. Modified Cork/SEBS Composites for 3D Printed Elastomers. Polym. Adv. Technol. 2022, 33, 1881–1891. [Google Scholar] [CrossRef]
- Saikrasun, S.; Yuakkul, D.; Amornsakchai, T. Thermo-Oxidative Stability and Remarkable Improvement in Mechanical Performance for Styrenic-Based Elastomer Composites Contributed from Silane-Treated Pineapple Leaf Fiber and Compatibilizer. Int. J. Plast. Technol. 2017, 21, 252–277. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Micro and Nanocrystalline Cellulose Derivatives of Lignocellulosic Biomass: A Review on Synthesis, Applications and Advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Principles of Cellulose Derivatization BT—Cellulose Derivatives: Synthesis, Structure, and Properties; Springer Series on Polymer and Composite Materials; Springer Nature: Basel, Switzerland, 2018. [Google Scholar]
- Dinesh; Kumar, B.; Kim, J. Mechanical and Dynamic Mechanical Behavior of the Lignocellulosic Pine Needle Fiber-Reinforced SEBS Composites. Polymers 2023, 15, 1225. [Google Scholar] [CrossRef]
- Murtaza, G. Ethylcellulose Microparticles: A Review. Acta Pol. Pharm. Drug Res. 2012, 69, 11–22. [Google Scholar]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of Impact Strength of Polylactide Blends with a Thermoplastic Elastomer Compatibilized with Biobased Maleinized Linseed Oil for Applications in Rigid Packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef]
- Gan, H.; Xu, J.; He, W. Study on the Properties of Cellulose Acetate Composite Synergistically Toughened by Glycerol Triacetate and Modified Nano Titania. J. Phys. Conf. Ser. 2023, 2539, 012065. [Google Scholar] [CrossRef]
- Garhwal, A.; Maiti, S.N. Influence of Styrene–ethylene–butylene–styrene (SEBS) Copolymer on the Short-Term Static Mechanical and Fracture Performance of Polycarbonate (PC)/SEBS Blends. Polym. Bull. 2016, 73, 1719–1740. [Google Scholar] [CrossRef]
- Ganguly, A.; Bhowmick, A.K. Effect of Polar Modification on Morphology and Properties of Styrene-(Ethylene-Co-Butylene)-Styrene Triblock Copolymer and Its Montmorillonite Clay-Based Nanocomposites. J. Mater. Sci. 2009, 44, 903–918. [Google Scholar] [CrossRef]
- Fernandes, L.C.; Correia, D.M.; Pereira, N.; Tubio, C.R.; Lanceros-Méndez, S. Highly Sensitive Transparent Piezoionic Materials and Their Applicability as Printable Pressure Sensors. Compos. Sci. Technol. 2021, 214, 108976. [Google Scholar] [CrossRef]
- Gupta, P.; Bera, M.; Maji, P.K. Nanotailoring of Sepiolite Clay with Poly [Styrene-b-(Ethylene-Co-Butylene)-b-Styrene]: Structure–property Correlation. Polym. Adv. Technol. 2017, 28, 1428–1437. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, Z.; Li, Y.; Luo, J.; Chen, Z.; Xia, J.; Liang, H.; Zhang, A. Order-Order, Lattice Disordering, and Order-Disorder Transition in SEBS Studied by Two-Dimensional Correlation Infrared Spectroscopy. Polymer 2010, 51, 4249–4258. [Google Scholar] [CrossRef]
- Senusi, N.A.; Mohd Shohaimi, N.A.; Halim, A.Z.A.; Shukr, N.M.; Abdul Razab, M.K.A.; Mohamed, M.; Mohd Amin, M.A.; Mokhtar, W.N.A.W.; Ismardi, A.; Abdullah, N.H.; et al. Preparation & Characterization of Microcrystalline Cellulose from Agriculture Waste. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012035. [Google Scholar]
- Gunduz, O.; Ahmad, Z.; Stride, E.; Edirisinghe, M. Continuous Generation of Ethyl Cellulose Drug Delivery Nanocarriers from Microbubbles. Pharm. Res. 2013, 30, 225–237. [Google Scholar] [CrossRef]
- Ghorani, B.; Russell, S.J.; Goswami, P. Controlled Morphology and Mechanical Characterisation of Electrospun Cellulose Acetate Fibre Webs. Int. J. Polym. Sci. 2013, 2013, 256161. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Demarquette, N.R. Blending and Morphology Control to Turn Hydrophobic SEBS Electrospun Mats Superhydrophilic. Langmuir 2015, 31, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, R.S.; Demarquette, N.R. Surface Properties Evolution in Electrospun Polymer Blends by Segregation of Hydrophilic or Amphiphilic Molecules. Eur. Polym. J. 2017, 89, 129–137. [Google Scholar] [CrossRef]
- Qi, J.; Chen, Y.; Zhang, W.T.; Li, L.; Huang, H.D.; Lin, H.; Zhong, G.J.; Li, Z.M. Imparting Cellulose Acetate Films with Hydrophobicity, High Transparency, and Self-Cleaning Function by Constructing a Slippery Liquid-Infused Porous Surface. Ind. Eng. Chem. Res. 2022, 61, 7962–7970. [Google Scholar] [CrossRef]
- Cantournet, S.; Desmorat, R.; Besson, J. Mullins Effect and Cyclic Stress Softening of Filled Elastomers by Internal Sliding and Friction Thermodynamics Model. Int. J. Solids Struct. 2009, 46, 2255–2264. [Google Scholar] [CrossRef]
- Lemes, A.P.; Talim, R.; Gomes, R.C.; Gomes, R.C. Preparation and Characterization of Maleic Anhydride Grafted Poly(Hydroxybutirate-CO-Hydroxyvalerate)—PHBV-g-MA. Mater. Res. 2016, 19, 229–235. [Google Scholar]
- Eid, M.; Abdel-Ghaffar, M.A.; Dessouki, A.M. Effect of Maleic Acid Content on the Thermal Stability, Swelling Behaviour and Network Structure of Gelatin-Based Hydrogels Prepared by Gamma Irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2009, 267, 91–98. [Google Scholar] [CrossRef]
- Bhuyan, K.; Dass, N.N. Thermal Studies of Copolymers of Styrene and Maleic Anhydride. J. Therm. Anal. 1989, 35, 2529–2533. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; Da Silva Barud, H.; De Assunção, R.M.N.; Da Silva Meireles, C.; Carvalho, G.O.; Filho, G.R.; Messaddeq, Y.; Ribeiro, S.J.L. Synthesis and Characterization of Microcrystalline Cellulose Produced from Bacterial Cellulose. J. Therm. Anal. Calorim. 2011, 106, 703–709. [Google Scholar] [CrossRef]
- Zyane, A.; Belfkira, A.; Brouillette, F.; Lucas, R.; Marchet, P. Microcrystalline Cellulose as a Green Way for Substituting BaTiO3 in Dielectric Composites and Improving Their Dielectric Properties. Cellul. Chem. Technol. 2015, 49, 783–787. [Google Scholar]
- Ribeiro, S.; Costa, P.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Lanceros-Méndez, S. Electrospun Styrene-Butadiene-Styrene Elastomer Copolymers for Tissue Engineering Applications: Effect of Butadiene/Styrene Ratio, Block Structure, Hydrogenation and Carbon Nanotube Loading on Physical Properties and Cytotoxicity. Compos. Part B Eng. 2014, 67, 30–38. [Google Scholar] [CrossRef]
- Sainorudin, M.H.; Abdullah, N.A.; Asmal Rani, M.S.; Mohammad, M.; Mahizan, M.; Shadan, N.; Abd Kadir, N.H.; Yaakob, Z.; El-Denglawey, A.; Alam, M. Structural Characterization of Microcrystalline and Nanocrystalline Cellulose from Ananas comosus L. Leaves: Cytocompatibility and Molecular Docking Studies. Nanotechnol. Rev. 2021, 10, 793–806. [Google Scholar] [CrossRef]










| Identification Sample | SEBS (wt%) | SEBS-g-MA (wt%) | EC (wt%) | CA (wt%) | MCC (wt%) |
|---|---|---|---|---|---|
| SEBS100 | 100 | 0 | 0 | 0 | 0 |
| SEBS90:EC10 | 90 | 0 | 10 | 0 | 0 |
| SEBS80:EC20 | 80 | 0 | 20 | 0 | 0 |
| SEBS70:EC30 | 70 | 0 | 30 | 0 | 0 |
| SEBS90:MCC10 | 90 | 0 | 0 | 0 | 10 |
| SEBS80:MCC20 | 80 | 0 | 0 | 0 | 20 |
| SEBS70:MCC30 | 70 | 0 | 0 | 0 | 30 |
| SEBS-g-MA100 | 0 | 100 | 0 | 0 | 0 |
| SEBS-g-MA90:EC10 | 0 | 90 | 10 | 0 | 0 |
| SEBS-g-MA80:EC20 | 0 | 80 | 20 | 0 | 0 |
| SEBS-g-MA70:EC30 | 0 | 70 | 30 | 0 | 0 |
| SEBS-g-MA90:CA10 | 0 | 90 | 0 | 10 | 0 |
| SEBS-g-MA80:CA20 | 0 | 80 | 0 | 20 | 0 |
| SEBS-g-MA70:CA30 | 0 | 70 | 0 | 30 | 0 |
| Blends | Morphology | Mechanical | Wettability | Dielectric | Citotoxicity |
|---|---|---|---|---|---|
| SEBS:EC | Regular oval-shaped aggregates | Hydrophobic | Enhanced | Not cytotoxic | |
| SEBS:CA | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| SEBS:MCC | Irregularly-shaped aggregates | Hydrophilic | Preserved | Not cytotoxic | |
| SEBS-g-MA:EC | Regular oval-shaped aggregates | Preserved tensile energy dissipation | Hydrophobic | Enhanced | Not cytotoxic |
| SEBS-g-MA:CA | Regular circular-shaped aggregates | Hydrophilic | Preserved | Not cytotoxic | |
| SEBS-g-MA:MCC | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajares, E.; Maestu, J.F.; Fernandez-de-Mendiola, I.; Silvan, U.; Costa, P.; Agirrezabal-Telleria, I.; Tubio, C.R.; Corona-Galván, S.; Lanceros-Mendez, S. Strategies for Improving Sustainability in the Development of High-Performance Styrenic Block Copolymers by Developing Blends with Cellulose Derivatives. Polymers 2024, 16, 856. https://doi.org/10.3390/polym16060856
Pajares E, Maestu JF, Fernandez-de-Mendiola I, Silvan U, Costa P, Agirrezabal-Telleria I, Tubio CR, Corona-Galván S, Lanceros-Mendez S. Strategies for Improving Sustainability in the Development of High-Performance Styrenic Block Copolymers by Developing Blends with Cellulose Derivatives. Polymers. 2024; 16(6):856. https://doi.org/10.3390/polym16060856
Chicago/Turabian StylePajares, Erika, Josu Fernández Maestu, Irati Fernandez-de-Mendiola, Unai Silvan, Pedro Costa, Iker Agirrezabal-Telleria, Carmen R. Tubio, Sergio Corona-Galván, and Senentxu Lanceros-Mendez. 2024. "Strategies for Improving Sustainability in the Development of High-Performance Styrenic Block Copolymers by Developing Blends with Cellulose Derivatives" Polymers 16, no. 6: 856. https://doi.org/10.3390/polym16060856
APA StylePajares, E., Maestu, J. F., Fernandez-de-Mendiola, I., Silvan, U., Costa, P., Agirrezabal-Telleria, I., Tubio, C. R., Corona-Galván, S., & Lanceros-Mendez, S. (2024). Strategies for Improving Sustainability in the Development of High-Performance Styrenic Block Copolymers by Developing Blends with Cellulose Derivatives. Polymers, 16(6), 856. https://doi.org/10.3390/polym16060856