Fluorinated Polydopamine Shell Decorated Fillers in Polytetrafluoroethylene Composite for Achieving Highly Reduced Coefficient of Thermal Expansion
Abstract
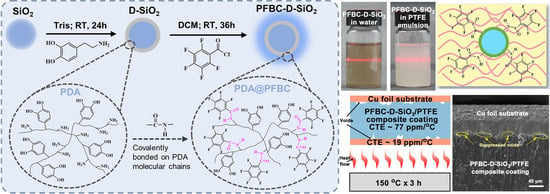
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PFBC-D-SiO2 Fillers and CCL
2.3. Characterization
3. Results and Discussion
3.1. Surface Chemical Structure Analysis of the Fillers
3.2. Wettability and Dispersibility of the Fillers
3.3. Interfacial Bonding State of the PTFE Composites
3.4. Mechanism of the Suppression of Thermal Debonding of PTFE Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alhaji, I.A.; Abbas, Z.; Zaid, M.H.M.; Zainuddin, N.; Khamis, A.M.; Lakin, I.I. Dielectric/thermal characteristics of recycled borosilicate glass-filled polytetrafluoroethylene. Mater. Chem. Phys. 2022, 290, 126529. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Cheng, W.; Zou, J.; Zhao, D. Progress on polymer composites with low dielectric constant and low dielectric loss for high-frequency signal transmission. Front. Mater. 2021, 8, 774843. [Google Scholar] [CrossRef]
- Wang, J.-C.; Shen, Z.-H.; Jiang, J.-Y.; Wang, J.; Zhang, X.; Shen, J.; Shen, Y.; Chen, W.; Chen, L.-Q.; Nan, C.-W. High-throughput finite-element design of dielectric composites for high-frequency copper clad laminates. Compos. Sci. Technol. 2022, 225, 109517. [Google Scholar] [CrossRef]
- Liu, F.; Jin, Y.; Li, J.; Jiang, W.; Zhao, W. Improved coefficient thermal expansion and mechanical properties of PTFE composites for high-frequency communication. Compos. Sci. Technol. 2023, 241, 110142. [Google Scholar] [CrossRef]
- Ren, J.; Yang, P.; Peng, Z.; Fu, X. Novel Al2Mo3O12-PTFE composites for microwave dielectric substrates. Ceram. Int. 2021, 47, 20867–20874. [Google Scholar] [CrossRef]
- Singh, V.; Singla, A.K.; Bansal, A. Influence of laser texturing along with PTFE topcoat on slurry and cavitation erosion resistance of HVOF sprayed VC coating. Surf. Coat. Technol. 2023, 470, 129858. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, Y.; Li, E.; Zhang, S. Ultra-high dielectric constant and thermal conductivity SrTiO3@VTMS/PB composite for microwave substrate application. Appl. Surf. Sci. 2023, 622, 156888. [Google Scholar] [CrossRef]
- Jin, W.; Li, A.; Li, Y.; Yu, Y.; Shen, J.; Zhou, J.; Chen, W. Enhancing high-frequency dielectric and mechanical properties of SiO2/PTFE composites from the interface fluorination. Ceram. Int. 2022, 48, 28512–28518. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, Y.; Yu, D.; Lin, H.; Wang, J.; Tang, B.; Li, E. Effects of compound coupling agents on the properties of PTFE/SiO2 microwave composites. J. Mater. Sci. Mater. Electron. 2017, 28, 3356–3363. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Q.; Deng, Y.; Li, X.; Luo, Z.; Zheng, B.; Dong, S. Assembly pattern of supramolecular hydrogel induced by lower critical solution temperature behavior of low-molecular-weight gelator. J. Am. Chem. Soc. 2020, 142, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Gao, J.; Yang, M.; Liu, Y.; Liu, Y.; Tang, X. Carbon layer-modified mesoporous silica supporter for PEG to improve the thermal properties of composite phase change material. J. Mater. Sci. 2021, 56, 5786–5801. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Zhang, C.; Luo, W.; Jiang, X. Enhanced thermal performance of phase-change materials supported by mesoporous silica modified with polydopamine/nano-metal particles for thermal energy storage. Renew. Energy 2021, 178, 118–127. [Google Scholar] [CrossRef]
- Li, Q.; Lin, G.; Zhang, S.; Wang, H.; Borah, J.; Jing, Y.; Liu, F. Conducting and stretchable emulsion styrene butadiene rubber composites using SiO2@Ag core-shell particles and polydopamine coated carbon nanotubes. Polym. Test. 2022, 115, 107722. [Google Scholar] [CrossRef]
- Ramirez-Soria, E.H.; León-Silva, U.; Lara-Ceniceros, T.E.; Bazán-Díaz, L.; Advíncula, R.C.; Bonilla-Cruz, J. Graphene oxide bifunctionalized with NH2/NH3+ and their outstanding-performance against corrosion. Appl. Surf. Sci. 2021, 561, 150048. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, D.; Zhou, J.; Shen, J.; Zhang, P.; Chen, W.; Zhou, J. Improved dispersion and interfacial interaction of SiO2@polydopamine fillers in polytetrafluoroethylene composites for reduced thermal expansion and suppressed dielectric deterioration. Ceram. Int. 2023, 49, 21492–21501. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. Role of silica mid-layer in thermal and chemical stability of hierarchical Fe3O4-SiO2-TiO2 nanoparticles for improvement of lead adsorption: Kinetics, thermodynamic and deep XPS investigation. Mater. Sci. Eng. B 2020, 262, 114690. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Wang, B.; Shi, Q.; Gao, J.; Cui, Z.; Wan, Y. Dopamine functionalization for improving crystallization behaviour of polyethylene glycol in shape-stable phase change material with silica fume as the matrix. J. Clean. Prod. 2019, 208, 951–959. [Google Scholar] [CrossRef]
- Ismail, I.; Wei, J.; Sun, X.; Zha, W.; Khalil, M.; Zhang, L.; Huang, R.; Chen, Z.; Shen, Y.; Li, F.; et al. Simultaneous improvement of the long-term and thermal stability of the perovskite solar cells using 2,3,4,5,6-pentafluorobenzoyl chloride (PFBC)-capped ZnO nanoparticles buffer layer. Sol. RRL 2020, 4, 2000289. [Google Scholar] [CrossRef]
- Li, Y.; Fan, M.; Wu, K.; Yu, F.; Chai, S.; Chen, F.; Fu, Q. Polydopamine coating layer on graphene for suppressing loss tangent and enhancing dielectric constant of poly(vinylidene fluoride)/graphene composites. Compos. Part A Appl. Sci. Manuf. 2015, 73, 85–92. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Jędrzak, A.; Szutkowski, K.; Grześkowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-based magnetic nanoparticles for cancer therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and its derivative surface chemistry in material science: A focused review for studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Zhang, D.; Xu, X.; Pan, M.; Haq, M.H.U.; Reda, A.T.; Xu, W. Barbituric and thiobarbituric acid-based UiO-66-NH2 adsorbents for iodine gas capture: Characterization, efficiency and mechanisms. J. Hazard. Mater. 2021, 416, 125835. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Shang, B.; Peng, B. Polydopamine based colloidal materials: Synthesis and applications. Chem. Rec. 2018, 18, 410–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jin, W.; Yin, Q.; Huang, J.; Huang, Z.; Fu, H.; Yuan, Y.; Zou, J.; Nie, J.; Zhang, Y. Ultrasensitive visual detection of Hg2+ ions via the Tyndall effect of gold nanoparticles. Chem. Commun. 2021, 57, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Deng, Z.; Huang, J.; Huang, Z.; Zhuang, M.; Yuan, Y.; Nie, J.; Zhang, Y. Highly sensitive colorimetric detection of a variety of analytes via the Tyndall effect. Anal. Chem. 2019, 91, 15114–15122. [Google Scholar] [CrossRef]
- Yu, K.; Niu, Y.; Xiang, F.; Zhou, Y.; Bai, Y.; Wang, H. Enhanced electric breakdown strength and high energy density of barium titanate filled polymer nanocomposites. J. Appl. Phys. 2013, 114, 174107. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, Y.; Yu, K.; Wang, Y.; Xiang, F.; Wang, H. Effect of the modifier structure on the performance of barium titanate/poly(vinylidene fluoride) nanocomposites for energy storage applications. ACS Appl. Mater. Inter. 2015, 7, 24168–24176. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.-J.; Marder, S.R.; Li, J.; Calame, J.P.; Perry, J.W. High energy density nanocomposites based on surface-modified BaTiO3 and a ferroelectric polymer. ACS Nano 2009, 3, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Hou, D.; Luo, X.; Han, C.; Fu, J.; Zeng, H.; Yu, F. Enhancing mechanical properties of polyelectrolyte complex nanofibers with graphene oxide nanofillers pretreated by polycation. Compos. Sci. Technol. 2016, 135, 128–136. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, Z.; Xu, X.; Shen, M.; Huang, G.; Bourbigot, S.; Liu, X.; Song, P. Mechanically robust and flame-retardant polylactide composites based on molecularly-engineered polyphosphoramides. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106317. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Lv, J. Preparing the SiC coated C/C composites with excellent mechanical and antioxidative properties using a buffer layer. J. Eur. Ceram. Soc. 2022, 42, 4162–4171. [Google Scholar] [CrossRef]
- Devendran, R.; Manivasakan, S.; Arumugam, R.; Livingstone, D.; Andhare, K.; Subramanian, B. Effect of EVA-TiO2 composite interfacial buffer layer in improving the bond strength between PMMA denture base and PDMS liner. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Shen, J.; Li, Q.; Qi, Y.; Chen, W. Ultra-low permittivity HSM/PTFE composites for high-frequency microwave circuit application. J. Mater. Sci. Mater. Electron. 2022, 33, 10096–10103. [Google Scholar] [CrossRef]
- Blumm, J.; Lindemann, A.; Meyer, M.; Strasser, C. Characterization of PTFE using advanced thermal analysis techniques. Int. J. Thermophys. 2010, 31, 1919–1927. [Google Scholar] [CrossRef]
- Mohan, T.P.; Kanny, K. Dynamic mechanical analysis of glass fiber reinforced epoxy filled nanoclay hybrid composites. Mater. Today Proc. 2023, 87, 235–245. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wang, H.; Li, E.; Yuan, Y. Investigation of PTFE-based ultra-low dielectric constant composite substrates with hollow silica ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 4550–4558. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Guo, J.; Yang, H.; Tong, J.; Zhang, Q. Modified BCZN particles filled PTFE composites with high dielectric constant and low loss for microwave substrate applications. Ceram. Int. 2020, 46, 7531–7540. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Yan, X.; Lu, G.; Xie, K.; Tong, J.; Meng, F. Functionalized Al2O3 fillers/glass fibers cloth/PTFE composites with excellent thermal properties. J. Mater. Sci. Mater. Electron. 2022, 33, 8815–8821. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Chen, X.; Hou, D.; Zhou, J.; Zhang, P.; Shen, J.; Zhou, J. Fluorinated Polydopamine Shell Decorated Fillers in Polytetrafluoroethylene Composite for Achieving Highly Reduced Coefficient of Thermal Expansion. Polymers 2024, 16, 987. https://doi.org/10.3390/polym16070987
Yu Y, Chen X, Hou D, Zhou J, Zhang P, Shen J, Zhou J. Fluorinated Polydopamine Shell Decorated Fillers in Polytetrafluoroethylene Composite for Achieving Highly Reduced Coefficient of Thermal Expansion. Polymers. 2024; 16(7):987. https://doi.org/10.3390/polym16070987
Chicago/Turabian StyleYu, Yuanying, Xiao Chen, Dajun Hou, Jingjing Zhou, Pengchao Zhang, Jie Shen, and Jing Zhou. 2024. "Fluorinated Polydopamine Shell Decorated Fillers in Polytetrafluoroethylene Composite for Achieving Highly Reduced Coefficient of Thermal Expansion" Polymers 16, no. 7: 987. https://doi.org/10.3390/polym16070987
APA StyleYu, Y., Chen, X., Hou, D., Zhou, J., Zhang, P., Shen, J., & Zhou, J. (2024). Fluorinated Polydopamine Shell Decorated Fillers in Polytetrafluoroethylene Composite for Achieving Highly Reduced Coefficient of Thermal Expansion. Polymers, 16(7), 987. https://doi.org/10.3390/polym16070987