Polyelectrolytes: Influence on Evaporative Self-Assembly of Particles and Assembly of Multilayers with Polymers, Nanoparticles and Carbon Nanotubes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Shell Fabrication of Silica Particles
2.3. Particle Arrays Construction
2.4. Gold Nanoparticles in Polyelectrolyte Films
2.5. Magnetic Nanoparticles in Polyelectrolyte Films
2.6. Fabrication of Films with Carbon Nanotubes
2.7. Characterization
3. Results and Discussion

3.1. Fabrication of Arrays of Silica Colloids and PEM Coated Silica Colloids


3.2. Gold Nanoparticles on Polyelectrolyte Multilayers

3.3. Magnetic Nanoparticles on Polyelectrolyte Multilayers

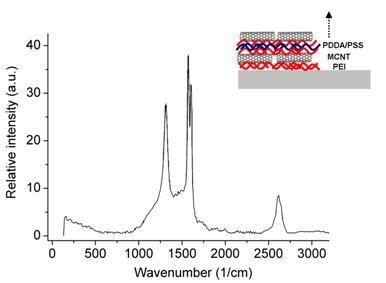
3.4. Carbon Nanotubes on Polyelectrolyte Multilayers

4. Conclusions
Acknowledgements
References
- Skirtach, A.G.; Kreft, O. Stimuli-sensitive nanotechnology for drug delivery. In Biotechnology: Pharmaceutical Aspects; de Velliers, M.M., Aramwit, P., Kwon, G.S., Eds.; Springer: New York, NY, USA, 2009; Volume 10; pp. 545–578. [Google Scholar]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Lvov, Y.; Decher, G.; Möhwald, H. Assembly, structural characterization, and thermal-behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulfate) and poly(ally amine). Langmuir 1993, 9, 481–486. [Google Scholar] [CrossRef]
- Caruso, F.; Furlong, D.N.; Ariga, K.; Ichinose, I.; Kunitake, T. Characterization of polyelectrolyte-protein multilayer films by atomic force microscopy, scanning electron microscopy, and Fourier transform infrared reflection-absorption spectroscopy. Langmuir 1998, 14, 4559–4565. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Mohwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Slocik, J.M.; Tsukruk, T.; Naik, R.R.; Tsukruk, V.V. Polyaminoacid-induced growth of metal nanoparticles on layer-by-layer templates. Chem. Mater. 2008, 20, 5822–5831. [Google Scholar] [CrossRef]
- Wandrey, C.; Hunkeler, D.; Wendler, U.; Jaeger, W. Counterion activity of highly charged strong polyelectrolytes. Macromolecules 2000, 33, 7136–7143. [Google Scholar] [CrossRef]
- Malinova, V.; Wandrey, C. Loading polyelectrolytes onto porous microspheres: Impact of molecular and electrochemical parameters. J. Phys. Chem. B 2007, 111, 8494–9501. [Google Scholar] [CrossRef] [PubMed]
- Jessel, N.; Alatar, F.; Lavalle, P.; Mutterer, J.; Decher, G.; Shaaf, P.; Voegel, J.C.; Odier, J. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv. Mater. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Benkriane-Jessel, N.; Schwinte, P.; Falvey, P.; Darcy, R.; Haikel, Y.; Shaaf, P.; Voegel, J.C.; Odier, J. Build-up of polypeptide multilayer coatings with anti-inflammatory properties based on the embedding of piroxicam-cyclodextrin complexes. Adv. Funct. Mater. 2004, 14, 174–182. [Google Scholar]
- Jessel, N.B.; Schwinte, P.; Donohue, R.; Lavalle, P.; Boulmedias, F.; Szalontai, P.; Darcy, R.; Voegel, J.C.; Odier, J. Pyridylamino-beta-cyclodextrin as a molecular chaperone for lipopolysaccharide embedded in a multilayered polyelectrolyte architecture. Adv. Funct. Mater. 2004, 14, 963–969. [Google Scholar] [CrossRef]
- Benkriane-Jessel, N.; Lavalle, P.; Hurbsh, E.; Holl, V.; Senger, B.; Haikel, Y.; Voegel, J.C.; Odier, J.; Shaaf, P. Short-time timing of the biological activity of functionalized polyelectrolyte multilayers. Adv. Funct. Mater. 2005, 15, 648–654. [Google Scholar] [CrossRef]
- Zhang, X.; Sharma, K.K.; Boeglin, M.; Ogier, J.; Mainard, D.; Voegel, J.C.; Mely, Y.; Benkirane-Jessel, N. Transfection ability and intracellular DNA pathway of nanostructured gene-delivery systems. Nano Lett. 2008, 13, 2432–2436. [Google Scholar] [CrossRef]
- Benkriane-Jessel, N.; Lavalle, P.; Meyer, F.; Audouin, F.; Frisch, B.; Shaaf, P.; Decher, G.; Voegel, J.C.; Odier, J. Control of monocyte morphology on and response to model surfaces for implants equipped with anti-inflammatory agents. Adv. Mater. 2004, 16, 1507–1511. [Google Scholar]
- Dierich, A.; Guen, E.L.; Messaddeq, N.; Stoltz, J.F.; Netter, P.; Shaaf, P.; Voegel, J.C.; Benkriane-Jessel, N. Bone formation mediated by synergy-acting growth factors embedded in a polyelectrolyte multilayer film. Adv. Mater. 2007, 19, 693–697. [Google Scholar] [CrossRef]
- Facca, S.; Cortez, C.; Mendoza-Palomares, C.; Messadeq, N.; Dierich, A.; Johnston, A.P.R.; Mainard, D.; Voegel, J.-C.; Caruso, F.; Benkirane-Jessel, N. Active multilayered capsules for in vivo bone formation. Proc. Nat. Acad. Sci. 2010, 107, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Alam, D.A.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haıkel, Y.; Gangloff, S.C.; Benkirane-Jessel, N. Nanostructured assemblies for dental application. ACS Nano 2010, 4, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Picart, C.; Lavalle, P.; Hubert, P.; Cuisinier, F.J.G.; Decher, G.; Schaaf, P.; Voegel, J.C. Buildup mechanism for poly(L-lysine)/hyaluronic acid films onto a solid surface. Langmuir 2001, 17, 7414–7424. [Google Scholar] [CrossRef]
- Mjahed, H.; Porcel, C.; Senger, B.; Chassepot, A.; Netter, P.; Gillet, P.; Decher, G.; Voegel, J.C.; Schaaf, P.; Benkirane-Jessel, N.; Boulmedias, F. Micro-stratified architectures based on successive stacking of alginate gel layers and poly(L-lysine)-hyaluronic acid multilayer films aimed at tissue engineering. Soft Matter 2008, 4, 1422–1429. [Google Scholar] [CrossRef]
- Boulmedais, F.; Ball, V.; Schwinte, P.; Frisch, B.; Schaaf, P.; Voegel, J.C. Buildup of exponentially growing multilayer polypeptide films with internal secondary structure. Langmuir 2003, 19, 440–445. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Delcea, M.; Mohwald, H.; Skirtach, A.G. Remote near-IR light activation of a hyaluronic acid/poly(l-lysine) multilayered film and film-entrapped microcapsules. ACS Appl. Mater. Interfaces 2009, 1, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Volodkin, D.V.; Mohwald, H. Bio-interfaces-Interaction of PLL/HA thick films with nanoparticles and microcapsules. Chem. Phys. Chem. 2010, 11, 822–829. [Google Scholar] [PubMed]
- Volodkin, D.V.; Madaboosi, N.; Blacklock, J.; Skirtach, A.G.; Moehwald, H. Surface-supported multilayers decorated with bio-active material aimed at light-triggered drug delivery. Langmuir 2009, 25, 14037–14043. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.G.; De Kokker, S.; Sukhorukov, G.B.; Kreft, O.; Parak, W.J.; Skirtach, A.G.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Polyelectrolyte microcapsules for biomedical applications. Soft Matter 2009, 5, 282–291. [Google Scholar] [CrossRef]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar] [PubMed]
- De Cock, L.J.; De Koker, S.; De Geest, B.G.; Grooten, J.; Vervaet, C.; Remon, J.P.; Sukhorukov, G.B.; Antipina, M.N. Polymeric multilayer capsules in drug delivery. Angew. Chem. Int. Ed. 2010, 49, 6954–6973. [Google Scholar]
- Rivera Gil, P.; Parak, W.J. Composite nanoparticles take aim at cancer. ACS Nano 2008, 2, 2200–2205. [Google Scholar]
- Rivera Gil, P.; del Mercato, L.L.; del-Pino, P.; Munoz-Javier, A.; Parak, W.J. Nanoparticle-modified polyelectrolyte capsules. Nano Today 2008, 3, 12–21. [Google Scholar]
- Matsuaki, M.; Akashi, M. Functional multilayered capsules for targeting and local drug delivery. Expert Opin. Drug Deliv. 2009, 6, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Mohwald, H.; Skirtach, A.G. Stimuli responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63. in press. [Google Scholar] [CrossRef]
- Orozco, V.H.; Kozlovskaya, V.; Kharlampieva, E.; López, B.L.; Tsukruk, V.V. Biodegradable self-reporting nanocomposite films of poly(lactic acid) nanoparticles engineered by layer-by-layer assembly. Polymer 2010, 51, 4127–4139. [Google Scholar] [CrossRef]
- Grohn, F. Soft matter nanoparticles with various shapes and functionalities can form through electrostatic self-assembly. Soft Matter 2010, 6, 4296–4302. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Kharlampieva, E.; Khanal, B.P.; Manna, P.; Zubarev, E.R.; Tsukruk, V.V. Ultrathin layer-by-layer hydrogels with incorporated gold nanorods as pH-sensitive optical materials. Chem. Mater. 2008, 20, 7474–7485. [Google Scholar] [CrossRef]
- Tokarev, I.; Tokareva, I.; Gopishetty, V.; Katz, E.; Minko, S. Specific biochemical-to-optical signal transduction by responsive thin hydrogel films loaded with noble metal nanoparticles. Adv. Mater. 2010, 22, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Bedard, M.F.; Munoz-Javier, A.; Mueller, R.; del Pino, P.; Fery, A.; Parak, W.J.; Skirtach, A.G.; Sukhorukov, G.B. On the mechanical stability of polymeric microcontainers functionalized with nanoparticles. Soft Matter 2009, 5, 148–155. [Google Scholar] [CrossRef]
- Delcea, M.; Schmidt, S.; Palankar, R.; Fernandes, P.A.L.; Fery, A.; Mohwald, H.; Skirtach, A.G. Mechanobiology: Correlation between mechanical stability of microcapsules studied by AFM and impact of cell-induced stresses. Small 2010. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Antipov, A.A.; Shchukin, D.G.; Sukhorukov, G.B. Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir 2004, 20, 6988–6992. [Google Scholar] [CrossRef] [PubMed]
- Radt, B.; Smith, T.A.; Caruso, F. Optically addressable nanostructured capsules. Adv. Mater. 2004, 16, 2184–2187. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Mohwald, H.; Sukhorukov, G.B. The role of metal nanoparticles in remote release of encapsulated materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Bedard, M.F.; Braun, D.; Sukhorukov, G.B.; Skirtach, A.G. Toward self-assembly of nanoparticles on polymeric microshells: Near-IR release and permeability. ACS Nano 2008, 2, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Bedard, M.F.; De Geest, B.G.; Moehwald, H.; Sukhorukov, G.B.; Skirach, A.G. Direction specific release from giant microgel-templated polyelectrolyte microcontainers. Soft Matter 2009, 5, 3927–3931. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Javier, AM; Kreft, O.; Kohler, K.; Alberola, A.P.; Mohwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef]
- Palankar, R.; Skirtach, A.G.; Kreft, O.; Bédard, M.; Garstka, M.; Gould, K.; Möhwald, H.; Sukhorukov, G.B.; Winterhalter, M.; Springer, S. Controlled intracellular release of peptides from microcapsules enhances antigen presentation on MHC class I molecules. Small 2009, 5, 2168–2176. [Google Scholar] [CrossRef]
- Lee, S.E.; Sasaki, D.Y.; Perroud, T.D.; Yoo, D.; Patel, K.D.; Lee, L.P. Biologically functional cationic phospholipid-gold nanoplasmonic carriers of RNA. J. Am. Chem. Soc. 2009, 131, 14066–14074. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I. Plasmonic heating assisted deposition of bare Au nanoparticles on titania nanoshells. J. Colloid Interface Sci. 2010, 351, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I. Writing, self-healing, and self-erasing on conductive pressure-sensitive adhesives. Small 2010, 6, 1679–1685. [Google Scholar] [CrossRef]
- Yu, J.; Javier, D.; Yaseen, M.A.; Nitin, N.; Richards-Kortum, R.; Anvari, B.; Wong, M.S. Self-assembly synthesis, tumor cell targeting, and photothermal capabilities of antibody-coated indocyanine green nanocapsules. J. Am. Chem. Soc. 2010, 132, 1929–1938. [Google Scholar] [CrossRef]
- Hrelescu, C.; Stehr, J.; Ringler, M.; Sperling, R.A.; Parak, W.J.; Klar, T.A.; Feldman, J. DNA melting in gold nanostove clusters. J. Phys. Chem. C 2010, 114, 7401–7411. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; de Abajo, F.J.G. Nanoscale control of optical heating in complex plasmonic systems. ACS Nano 2010, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Terentyuk, G.S.; Maslyakova, G.N.; Suleymanova, L.V.; Khlebtsov, N.G.; Khlebtsov, B.N.; Akchurin, G.G.; Maksimova, I.L.; Tuchin, V.V. Laser-induced tissue hyperthermia mediated by gold nanoparticles: Toward cancer phototherapy. J. Biomed. Opt. 2009, 14, 021016. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.E.; Hansen, E.; Lukianova-Hleb, E.Y.; Hafner, J.H.; Lapotko, D.O. Optically guided controlled release from liposomes with tunable plasmonic nanobubbles. J. Control. Release 2010, 144, 151–158. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Shao, R.P.; Wei, X.; Gupta, S.; Li, C. Near-infrared light triggers release of paclitaxel from biodegradable microspheres: photothermal effect and enhanced antitumor activity. Small 2010, 6, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.Y.; Li, C.M. Direct modulation of localized surface plasmon coupling of Au nanoparticles on solid substrates via weak polyelectrolyte-mediated layer-by-layer self assembly. Langmuir 2009, 25, 7578–7585. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.G.; McKnight, M.D.; Sharp, A.C.; Hestekin, J.A.; Roper, D.K. Gold nanoparticles allow optoplasmonic evaporation from open silica cells with a logarithmic approach to steady-state thermal profiles. J. Phys. Chem. C 2010, 114, 10132–10139. [Google Scholar] [CrossRef]
- Barman, A.K.; Verma, S. Sunlight mediated disruption of peptide-based soft structures decorated with gold nanoparticles. Chem. Commun. 2010, 46, 6992–6994. [Google Scholar] [CrossRef]
- Gorin, D.A.; Portnov, S.A.; Inozemtseva, O.A.; Luklinska, Z.; Yashchenok, A.M.; Pavlov, A.M.; Skirach, A.G.; Mohwald, H.; Sukhorukov, G.B. Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Nakamura, M.; Koumoto, K. Magnetoresponsive smart capsules formed with polyelectrolytes, lipid bilayers and magnetic nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ko, H.; Tsukruk, V.V. Strain-sensitive Raman modes of carbon nanotubes in deflecting freely suspended nanomembranes. Adv. Mater. 2005, 17, 2127–2131. [Google Scholar] [CrossRef]
- Ko, H.; Jiang, C.; Shulha, H.; Tsukruk, V.V. Carbon nanotube arrays encapsulated into freely suspended flexible films. Chem. Mater. 2005, 17, 2490–2493. [Google Scholar] [CrossRef]
- Patine, S.J.; Okawa, D.; Zettl, A.; Frechet, J.M.J. Chemicals on demand with phototriggerable microcapsules. J. Am. Chem. Soc. 2009, 131, 13586–13587. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Yang, H.M.; Koo, H.Y.; Lee, H.J.; Lee, Y.B.; Bae, T.S.; Jeon, I.C. Smart microcapsules encapsulating reconfigurable carbon nanotube cores. Adv. Funct. Mater. 2010, 20, 820–825. [Google Scholar] [CrossRef]
- Yashchenok, A.M.; Bratashov, D.N.; Gorin, D.A.; Lomova, M.V.; Pavlov, A.M.; Sapelkin, A.V.; Shim, B.S.; Khomutov, G.B.; Kotov, N.A.; Sukhorukov, G.B.; Möhwald, H.; Skirtach, A.G. Carbon nanotubes on polymeric microcapsules: Free-standing structures and point-wise laser openings. Adv. Funct. Mater. 2010, 20, 3136–3142. [Google Scholar] [CrossRef]
- Paunov, V.N.; Panhuis, M.I.H. Fabrication of carbon nanotube-based microcapsules by a colloid templating technique. Nano Technol. 2005, 16, 1522–1525. [Google Scholar]
- Ji, Q.M.; Yoon, S.B.; Hill, J.P.; Vinu, A.; Yu, J.S.; Ariga, K.S. Layer-by-layer films of dual-pore carbon capsules with designable selectivity of gas adsorption. J. Am. Chem. Soc. 2009, 131, 4220–4221. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Skirtach, A.G.; Seki, T.; Yagai, S.; Li, H.G.; Mohwald, H.; Nakanishi, T. Assembly of fullerene-carbon nanotubes: temperature indicator for photothermal conversion. J. Am. Chem. Soc. 2010, 132, 8566–8568. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.H.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Caruso, F. Fabrication of polyaniline inverse opals via templating ordered colloidal assemblies. Adv. Mater. 2001, 13, 350–35. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Knobbe, C.B.; Ozin, G.A. A step towards optically encoded silver release in 1D photonic crystals. Small 2009, 5, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, A.C.; Puzzo, D.P.; Manners, I.; Ozin, G.A. Photonic-crystal full-colour displays. Nat. Photonic. 2007, 1, 468–472. [Google Scholar] [CrossRef]
- Min, W.L.; Jiang, P.; Jiang, B. Large-scale assembly of colloidal nanoparticles and fabrication of periodic subwavelength structures. Nano Technol. 2008, 19, 475604. [Google Scholar]
- Jiang, P.; McFarland, M.J. Large-scale fabrication of wafer-size colloidal crystals, macroporous polymers and nanocomposites by spin-coating. J. Am. Chem. Soc. 2004, 126, 13778–13786. [Google Scholar] [CrossRef] [PubMed]
- Braun, P.V.; Wiltzius, P. Microporous materials—Electrochemically grown photonic crystals. Nature 1999, 402, 603–604. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Sun, L.G.; Han, G.Z.; Gu, Z.Z. Optical switching of a birefringent photonic crystal. Adv. Mater. 2008, 20, 3601–3604. [Google Scholar] [CrossRef]
- Bardosova, M.; Pemble, M.E.; Povey, I.M.; Tredgold, R.H. The Langmuir-Blodgett approach to making colloidal photonic crystals from silica spheres. Adv. Mater. 2010, 22, 3104–3124. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Byun, M.; Lin, Z. Robust self-assembly of highly ordered complex structures by controlled evaporation of confined microfluids. Angew. Chem. Int. Ed. 2009, 48, 512–516. [Google Scholar] [CrossRef]
- Briseno, A.L.; Han, S.; Rauda, I.E.; Zhou, F.M.; Toh, C.S.; Nemanick, E.J.; Lewis, N.S. Electrochemical polymerization of aniline monomers infiltrated into well-ordered truncated eggshell structures of polyelectrolyte multilayers. Langmuir 2004, 20, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Balazs, A.C. Designing microcapsule arrays that propagate chemical signals. Phys. Rev. E 2010, 82, 021801. [Google Scholar] [CrossRef]
- Park, J.Y.; Ponnapati, R.; Taranekar, P.; Advincula, R.C. Carbazole peripheral poly(benzyl ether) dendrimers at the air-water interface: Electrochemical cross-linking and electronanopatterning. Langmuir 2010, 26, 6167–6176. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; De Geest, B.G.; Du Prez, F.E. On-demand click functionalization of polyurethane films and foams. Polymer 2009, 50, 5362–5367. [Google Scholar] [CrossRef]
- Alves, N.M.; Picart, C.; Mano, J.F. Self assembling and crosslinking of polyelectrolyte multilayer films of chitosan and alginate studied by QCM and IR spectroscopy. Macromol. BioSci. 2009, 9, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, I.; Minko, S. Stimuli-responsive porous hydrogels at interfaces for molecular filtration, separation, controlled release, and gating in capsules and membranes. Adv. Mater. 2010, 22, 3446–3462. [Google Scholar] [CrossRef] [PubMed]
- Staedler, B.; Chandrawati, R.; Price, A.D.; Chong, S.F.; Breheney, S.F.; Postma, A.; Connal, L.A.; Zelikin, A.N.; Caruso, F. A microreactor with thousands of subcompartments: enzyme-loaded liposomes within polymer capsules. Angew. Chem. Int. Ed. 2009, 48, 4359–4362. [Google Scholar] [CrossRef]
- Yashchenok, A.M.; Delcea, M.; Videnova, K.; Jares-Erijman, E.A.; Jovin, T.M.; Konrad, M.; Mohwald, H.; Skirtach, A.G. Enzyme reaction in the pores of CaCO3 particles upon ultrasound disruption of attached substrate-filled liposomes. Angew. Chem. Int. Ed. 2010, 49, 8116–8120. [Google Scholar] [CrossRef]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-calcium carbonate coprecipitation: A tool for protein encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Gittins, D.; Caruso, F. Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew Chem. Int. Ed. 2001, 40, 3001–3004. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Reinhardt, K.A.; Kern, W. Handbook of Semiconductor Wafer Cleaning Technology; William Andrew Inc.: New York, NY, USA, 2007. [Google Scholar]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Sukhorukov, G.B. Nanoparticles distribution control by polymers: Aggregates versus nonaggregates. J. Phys. Chem. C 2007, 111, 555–564. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Bedard, M.F.; Bukreeva, T.V.; Sukhorukov, G.B.; Mohwald, H.; Skirtach, A.G. Nanoparticles on polyelectrolytes at low concentration: controlling concentration and size. J. Phys. Chem. C 2010, 114, 1996–2002. [Google Scholar] [CrossRef]
- De Geest, B.G.; Skirtach, A.G.; De Beer, T.R.M.; Sukhorukov, G.B.; Bracke, L.; Baeyens, W.R.G.; Demeester, J.; De Smedt, S.C. Stimuli-responsive multilayered hybrid nanoparticle/polyelectrolyte capsules. Macromol. Rapid Commun. 2007, 28, 88–95. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Boul, P.; Ericson, L.M.; Huffman, C.; Wang, Y.H.; Haroz, E.; Kuper, C.; Tour, J.; Ausman, K.D.; Smalley, R.E. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 2001, 342, 265–271. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L.; Jackel, F.; Klar, T.A.; Feldmann, J. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhang, L.Y.; Qian, H.Q.; Li, R.T.; Jiang, X.Q.; Liu, B.R. Multifunctional nanocarriers for cell imaging, drug delivery, and near-ir photothermal therapy. Langmuir 2010, 26, 5428–5434. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Rege, K.; Heys, J.J. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano 2010, 4, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Gorin, D.A.; Portnov, S.A.; Inozemtseva, O.A.; Luklinska, Z.; Yashchenok, A.M.; Pavlov, A.M.; Skirach, A.G.; Mohwald, H.; Sukhorukov, G.B. Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, A.; Cui, D.; Dubreuil, F.; De Geest, B.G.; De Cock, L.J.; Picart, C.; Auzely-Velty, R. Designing hyaluronic acid-based layer-by-layer capsules as a carrier for intracellular drug delivery. Biomacromolecules 2010, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Yashchenok, A.; Videnova, K.; Kreft, O.; Mohwald, H.; Skirtach, A.G. Multicompartmental micro- and nanocapsules: Hierarchy and applications in biosciences. Micromol. Biosci. 2010, 10, 465–474. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marchenko, I.; Yashchenok, A.; German, S.; Inozemtseva, O.; Gorin, D.; Bukreeva, T.; Mohwald, H.; Skirtach, A. Polyelectrolytes: Influence on Evaporative Self-Assembly of Particles and Assembly of Multilayers with Polymers, Nanoparticles and Carbon Nanotubes. Polymers 2010, 2, 690-708. https://doi.org/10.3390/polym2040690
Marchenko I, Yashchenok A, German S, Inozemtseva O, Gorin D, Bukreeva T, Mohwald H, Skirtach A. Polyelectrolytes: Influence on Evaporative Self-Assembly of Particles and Assembly of Multilayers with Polymers, Nanoparticles and Carbon Nanotubes. Polymers. 2010; 2(4):690-708. https://doi.org/10.3390/polym2040690
Chicago/Turabian StyleMarchenko, Irina, Alexey Yashchenok, Sergey German, Olga Inozemtseva, Dmitry Gorin, Tatiana Bukreeva, Helmuth Mohwald, and Andre Skirtach. 2010. "Polyelectrolytes: Influence on Evaporative Self-Assembly of Particles and Assembly of Multilayers with Polymers, Nanoparticles and Carbon Nanotubes" Polymers 2, no. 4: 690-708. https://doi.org/10.3390/polym2040690
APA StyleMarchenko, I., Yashchenok, A., German, S., Inozemtseva, O., Gorin, D., Bukreeva, T., Mohwald, H., & Skirtach, A. (2010). Polyelectrolytes: Influence on Evaporative Self-Assembly of Particles and Assembly of Multilayers with Polymers, Nanoparticles and Carbon Nanotubes. Polymers, 2(4), 690-708. https://doi.org/10.3390/polym2040690