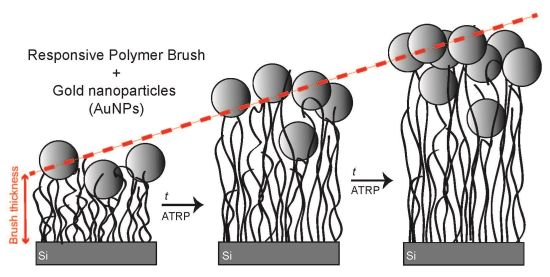
Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution
Abstract
:1. Introduction
2. Experimental Section
2.1. Instruments

2.2. Synthesis

3. Results and Discussion
3.1. Results
3.1.1. Initiator Self-Assembled Monolayer (SAM)
3.1.2. PDMAEMA Brushes



3.1.3. Brush/AuNP-13 Hybrids



| XRR Layer 1 | XRR Layer 2 | XRR Layer 3 | |||||
|---|---|---|---|---|---|---|---|
| h1 (nm) | ρe,1 (Å−3) | h2 (nm) | ρe,2 (Å−3) | h3 (nm) | ρe,3 (Å−3) | ||
| 9 | 0.79 | 9 | 1.56 | 4 | 0.25 | ||
| 47 | 0.66 | 9 | 1.86 | 3 | 0.21 | ||
| 109 | 0.62 | 9 | 2.11 | 3 | 0.23 | ||
| 156 | 0.56 | 7 | 2.28 | 4 | 0.41 | ||
| PDMAEMA | PDMAEMA/AuNP-13 | ||
|---|---|---|---|
| hElli (nm) | hAFM (nm) | hXRR (nm) | |
| 10 ± 0.3 | 20 ± 1 | 22 | |
| 41 ± 2 | 51 ± 2 | 58 | |
| 94 ± 2 | 124 ± 3 | 121 | |
| 128 ± 2 | 164 ± 4 | 167 | |

= ϕiρe,i + (1 − ϕi) ρe,j



3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andruzzi, L.; Senaratne, W.; Hexemer, A.; Sheets, E.D.; Ilic, B.; Kramer, E.J.; Baird, B.; Ober, C.K. Oligo(ethylene glycol) containing polymer brushes as bioselective surfaces. Langmuir 2005, 21, 2495–504. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Tam, T.K.; Pita, M.; Tokarev, I.; Katz, E.; Minko, S. Switchable selectivity for gating ion transport with mixed polyelectrolyte brushes: Approaching ‘smart’ drug delivery systems. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- An, S.W.; Thirtle, P.N.; Thomas, R.K.; Baines, F.L.; Billingham, N.C.; Armes, S.P.; Penfold, J. Structure of a diblock copolymer adsorbed at the hydrophobic solid/aqueous interface: Effects of charge density on a weak polyelectrolyte brush. Macromolecules 1999, 32, 2731–2738. [Google Scholar] [CrossRef]
- Houbenov, N.; Minko, S.; Stamm, M. Mixed polyelectrolyte brush from oppositely charged polymers for switching of surface charge and composition in aqueous environment. Macromolecules 2003, 36, 5897–5901. [Google Scholar] [CrossRef]
- Geoghegan, M.; Ruiz-Perez, L.; Dang, C.C.; Parnell, A.J.; Martin, S.J.; Howse, J.R.; Jones, R.A.L.; Golestanian, R.; Topham, P.D.; Crook, C.J.; et al. The pH-induced swelling and collapse of a polybase brush synthesized by atom transfer radical polymerization. Soft Matter 2006, 2, 1076–1080. [Google Scholar] [CrossRef]
- Plamper, F.A.; Schmalz, A.; Ballauff, M.; Mu, A.H.E. Tuning the thermoresponsiveness of weak polyelectrolytes by pH and light: Lower and upper critical-solution temperature of poly(N,N-dimethylaminoethyl methacrylate). J. Am. Chem. Soc. 2007, 129, 14538–14539. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, K.; Aulich, D.; Ionov, L.; Esser, N.; Eichhorn, K.J.; Motornov, M.; Stamm, M.; Minko, S. Chemical and structural changes in a pH-responsive mixed polyelectrolyte brush studied by infrared ellipsometry. Langmuir 2009, 25, 10987–10991. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wildes, A.; Titmuss, S. Structure of pH-responsive polymer brushes grown at the gold-water interface: Dependence on grafting density and temperature. Macromolecules 2012, 45, 305–312. [Google Scholar] [CrossRef]
- Balamurugan, S.; Mendez, S.; Balamurugan, S.S.; O’Brie, M.J.; Lopez, G.P. Thermal response of poly (N-isopropylacrylamide ) brushes probed by surface plasmon resonance. Langmuir 2003, 19, 2545–2549. [Google Scholar] [CrossRef]
- Yim, H.; Kent, M.S.; Mendez, S.; Balamurugan, S.S.; Balamurugan, S.; Lopez, G.P.; Satija, S. Temperature-dependent conformational change of PNIPAM grafted chains at high surface density in water. Macromolecules 2004, 37, 1994–1997. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, G. Collapse and swelling of thermally sensitive poly(N-isopropylacrylamide) brushes monitored with a quartz crystal microbalance. J. Phys. Chem. B 2005, 109, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, M.; Johannsmann, D.; Ruehe, J. Synthesis and swelling behavior of a weak polyacid brush. J. Chem. Phys. 2002, 117, 4988. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Sieval, A.B.; Fleer, G.J.; Stuart, M.A.C. Polyacrylic acid brushes: Surface pressure and salt-induced swelling. Langmuir 2000, 16, 8324–8333. [Google Scholar] [CrossRef]
- Mouri, E.; Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H.; Torikai, N. Effect of salt concentration on the nanostructure of weak polyacid brush in the amphiphilic polymer monolayer at the air/water interface. Langmuir 2004, 20, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Swelling of poly(methacrylic acid) brushes: Influence of monovalent salts in the environment. Macromolecules 2005, 38, 4855–4860. [Google Scholar] [CrossRef]
- Sanjuan, S.; Perrin, P.; Pantoustier, N.; Tran, Y. Synthesis and swelling behavior of pH-responsive polybase brushes. Langmuir 2007, 23, 5769–5778. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, M.; Ruehe, J. Scaling laws for the swelling of neutral and charged polymer brushes in good solvents. Macromolecules 2002, 35, 499–507. [Google Scholar] [CrossRef]
- Ross, R.S.; Pincus, P. The polyelectrolyte brush: Poor solvent. Macromolecules 1992, 25, 2177–2183. [Google Scholar] [CrossRef]
- Weir, M.P.; Heriot, S.Y.; Martin, S.J.; Parnell, A.J.; Holt, S.A.; Webster, J.R.P.; Jones, R.A.L. Voltage-induced swelling and deswelling of weak polybase brushes. Langmuir 2011, 27, 11000–11007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, M.; Uhlmann, P.; Simon, F.; Oertel, U.; Stamm, M. Gold nanoparticles immobilized on stimuli responsive polymer brushes as nanosensors. Macromolecules 2008, 41, 8152–8158. [Google Scholar] [CrossRef]
- Mitsuishi, M.; Koishikawa, Y.; Tanaka, H.; Sato, E.; Mikayama, T.; Matsui, J.; Miyashita, T. Nanoscale actuation of thermoreversible polymer brushes coupled with localized surface plasmon resonance of gold nanoparticles. Langmuir 2007, 23, 7472–7474. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, I.; Minko, S.; Fendler, J.H.; Hutter, E. Nanosensors based on responsive polymer brushes and gold nanoparticle enhanced transmission surface plasmon resonance spectroscopy. J. Am. Chem. Soc. 2004, 126, 15950–15951. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, D.; De, C.; Alarco, H.; Peters, V.; Smith, J.R.; Alexander, C. Poly(N-isopropylacrylamide) copolymers: Effect of phase transitions on protein and bacterial attachment. Langmuir 2003, 19, 2888–2899. [Google Scholar] [CrossRef]
- Hayashi, H.; Kono, K.; Takagishi, T. Temperature-controlled release property of phospholipid vesicles bearing a thermo-sensitive polymer. Biochim. Biophys. Acta 1996, 1280, 127–134. [Google Scholar]
- Ista, L.K.; Pérez-luna, V.H.; López, G.P. Surface-grafted, environmentally sensitive polymers for biofilm release. Appl. Environ. Microbiol. 1999, 65, 1603–1609. [Google Scholar] [PubMed]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Stayton, P.S.; Shimoboji, T.; Long, C.; Chilkoti, A.; Chen, G.; Harris, J.M.; Hoffmann, A.S. Control of protein ligand recognition using a stimuli responsive polymer. Nature 1995, 378, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, S.; Arifuzzaman, S.; Genzer, J.; Vaia, R.A. Capture and release. ACS Nano 2009, 3, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv. Rev. 2001, 46, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo-and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- De Las Heras Alarcon, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.R.; Genzer, J.; Chaney, B.N.; Sugg, H.W.; Liebmann-Vinson, A. Controlling the assembly of nanoparticles using surface grafted molecular and macromolecular gradients. Nanotechnology 2003, 14, 1145–1152. [Google Scholar] [CrossRef]
- Kim, J.; O’Shaughnessy, B. Morphology selection of nanoparticle dispersions by polymer media. Phys. Rev. Lett. 2002, 89, 238301. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; O’Shaughnessy, B. Nanoinclusions in dry polymer brushes. Macromolecules 2006, 39, 413–425. [Google Scholar] [CrossRef]
- Biesalski, M.; Ruehe, J. Swelling of a polyelectrolyte brush in humid air. Langmuir 2000, 11, 1943–1950. [Google Scholar] [CrossRef]
- Balzer, B.N.; Micciulla, S.; Dodoo, S.; Zerball, M.; Gallei, M.; Rehahn, M.; von Klitzing, R.; Hugel, T. Adhesion property profiles of supported thin polymer films. ACS Appl. Mater. Interfaces 2013, 5, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Heavens, O.S. Optical properties of thin films. Rep. Prog. Phys. 1960, 23, 1–65. [Google Scholar] [CrossRef]
- Olivier, A.; Meyer, F.; Raquez, J.M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 37, 157–181. [Google Scholar] [CrossRef]
- Patten, T.E.; Xia, J.; Abernathy, T.; Matyjaszewski, K. Polymers with very low polydispersities from atom transfer radical polymerization. Science 1996, 272, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.L.; Khan, M.A.; de Paz Banez, M.V.; Wang, X.S.; Armes, S.P. Controlled polymerization of 2-hydroxyethyl methacrylate by ATRP at ambient temperature. Macromolecules 2001, 34, 3155–3158. [Google Scholar] [CrossRef]
- Xia, J.; Gaynor, S.G.; Matyjaszewski, K. Acrylates at ambient temperature. Macromolecules 1998, 31, 5958–5959. [Google Scholar] [CrossRef]
- Jones, B.D.M.; Huck, W.T.S. Controlled surface-initiated polymerizations. Adv. Mater. 2001, 13, 1256–1259. [Google Scholar] [CrossRef]
- Huang, W.; Kim, J.B.; Bruening, M.L.; Baker, G.L. Functionalization of surfaces by water-accelerated atom-transfer radical polymerization of hydroxyethyl methacrylate and subsequent derivatization. Macromolecules 2002, 35, 1175–1179. [Google Scholar] [CrossRef]
- Xia, J.; Matyjaszewski, K. Controlled /living radical polymerization. Atom transfer radical polymerization using multidentate amine ligands. Macromolecules 1997, 30, 7697–7700. [Google Scholar]
- Tang, W.; Matyjaszewski, K. Effect of ligand structure on activation rate constants in ATRP. Macromolecules 2006, 39, 4953–4959. [Google Scholar] [CrossRef]
- Paik, H.J.; Horwitz, C.P. Tridentate nitrogen-based ligands in Cu-based ATRP: A structure—Activity study. Macromolecules 2001, 34, 430–440. [Google Scholar] [CrossRef]
- Tang, W.; Matyjaszewski, K. Effects of initiator structure on activation rate constants in ATRP. Macromolecules 2007, 40, 1858–1863. [Google Scholar] [CrossRef]
- Xia, J.; Matyjaszewski, K. Controlled/“living” radical polymerization. Homogeneous reverse atom transfer radical polymerization using AIBN as the initiator. Macromolecules 1997, 30, 7692–7696. [Google Scholar]
- Tang, W.; Nanda, A.K.; Matyjaszewski, K. Effect of [pyridylmethanimine]/[CuI] ratio, ligand, solvent and temperature on the activation rate constants in atom transfer radical polymerization. Macromol. Chem. Phys. 2005, 206, 1171–1177. [Google Scholar] [CrossRef]
- Kim, J.B.; Huang, W.; Miller, M.D.; Baker, G.L.; Bruening, M.L. Kinetics of surface-initiated atom transfer radical polymerization. J. Polym. Sci. A 2003, 41, 386–394. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Nanda, A.K.; Tang, W. Effect of [CuII] on the rate of activation in ATRP. Macromolecules 2005, 38, 2015–2018. [Google Scholar] [CrossRef]
- Nanda, A.K.; Matyjaszewski, K. Effect of [PMDETA]/[Cu(I)] ratio, monomer, solvent, counterion, ligand, and alkyl bromide on the activation rate constants in atom transfer radical polymerization. Macromolecules 2003, 36, 1487–1493. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Shipp, D.A.; Wang, J.L.; Grimaud, T.; Patten, T.E. Utilizing halide exchange to improve control of atom transfer radical polymerization. Macromolecules 1998, 31, 6836–6840. [Google Scholar] [CrossRef]
- Laurent, P.; Souharce, G.; Duchet-Rumeau, J.; Portinha, D.; Charlot, A. ‘Pancake’ vs. brush-like regime of quaternizable polymer grafts: An efficient tool for nano-templating polyelectrolyte self-assembly. Soft Matter 2012, 8, 715–725. [Google Scholar]
- Bain, E.D.; Dawes, K.; Özçam, A.E.; Hu, X.; Gorman, C.B.; Šrogl, J.; Genzer, J. Surface-initiated polymerization by means of novel, stable, non-ester-based radical initiator. Macromolecules 2012, 45, 3802–3815. [Google Scholar] [CrossRef]
- Cheng, N.; Azzaroni, O.; Moya, S.; Huck, W.T.S. The effect of [CuI]/[CuII] ratio on the kinetics and conformation of polyelectrolyte brushes by atom transfer radical polymerization. Macromol. Rapid Commun. 2006, 27, 1632–1636. [Google Scholar] [CrossRef]
- Karg, M.; Schelero, N.; Oppel, C.; Gradzielski, M.; Hellweg, T.; von Klitzing, R. Versatile phase transfer of gold nanoparticles from aqueous media. Chemistry 2011, 17, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Enustun, B.V.; Turkevich, J. Coagulation of colloidal gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; et al. Polymers at interfaces: Using atom transfer radical polymerization in the controlled growth of homopolymers and block copolymers from silicon surfaces in the absence of untethered sacrificial initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar]
- Rauch, S.; Uhlmann, P.; Eichhorn, K.J. In situ spectroscopic ellipsometry of pH-responsive polymer brushes on gold substrates. Anal. Bioanal. Chem. 2013, 405, 9061–9069. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Christau, S.; Thurandt, S.; Yenice, Z.; Von Klitzing, R. Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution. Polymers 2014, 6, 1877-1896. https://doi.org/10.3390/polym6071877
Christau S, Thurandt S, Yenice Z, Von Klitzing R. Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution. Polymers. 2014; 6(7):1877-1896. https://doi.org/10.3390/polym6071877
Chicago/Turabian StyleChristau, Stephanie, Stefan Thurandt, Zuleyha Yenice, and Regine Von Klitzing. 2014. "Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution" Polymers 6, no. 7: 1877-1896. https://doi.org/10.3390/polym6071877
APA StyleChristau, S., Thurandt, S., Yenice, Z., & Von Klitzing, R. (2014). Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution. Polymers, 6(7), 1877-1896. https://doi.org/10.3390/polym6071877