Synthesis and Structure Characterization of Phenol-Urea-Formaldehyde Resins in the Presence of Magnesium Oxide as Catalyst
Abstract
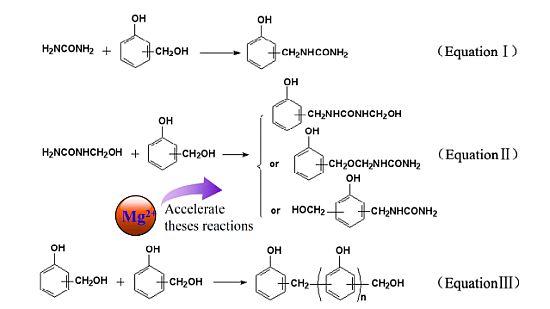
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Resins Synthesis
2.3. Properties Characterization
| Resins | PUF0 | PUF1 | PUF2 | PUF3 | PUF4 | PUF5 | PUF6 | PUF7 | PUF8 | PUF9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Nonvolatile solids (%) | 49.1 | 49.1 | 49.2 | 48.7 | 48.9 | 49.0 | 48.6 | 48.8 | 48.9 | 48.5 |
| pH | 11.6 | 11.8 | 11.5 | 11.4 | 10.9 | 11.5 | 12.1 | 11.6 | 11.5 | 11.7 |
| Viscosity (mPa·s) | 282 | 276 | 257 | 264 | 268 | 251 | 282 | 274 | 260 | 249 |
| Cure time (s) | 304 | 285 | 266 | 257 | 293 | 266 | 243 | 289 | 266 | 275 |
2.4. Liquid 13C-NMR Measurement
3. Results and Discussion
3.1. Chemical Structure


| Chemical Shifts (ppm) | Assignment of Groups |
|---|---|
| 163.1–163.5 | Unreacted urea |
| 159.6–162.8 | Mono-, di-, and tri-substituted ureas |
| 152.9–159.1 | Phenoxy region |
| 125.6–137.1 | Substituted aromatic carbons |
| 116.1–123.5 | Free aromatic carbons |
| 80.0–91.0 | Reactive formaldehyde adducts |
| 69.7–73.6 | Methylol groups of urea units |
| 68.3–69.5 | Methylene ether between ures units |
| 63.5–66.3 | Phenolic para methylol |
| 60.3–63.0 | Phenolic ortho methylol |
| 54.6–55.4 | Co-condensed methylene at phenolic para position |
| 49.6 | Methanol |
| 46.1–48.2 | Co-condensed methylene at phenolic ortho position |
| 39–42 | para-para methylene bridges |
| 34–36 | ortho-para methylene bridges |
| 29–30 | ortho-ortho methylene bridges |
3.2. Synthesis
| Resin No a | Synthesis Parameters | Para/Ortho (–CH2OH) c | Ortho/Para (Ph–CH2–Urea) d | –CH2OH/–CH2– e | –H/–CHOH2 f | Unreacted Urea | |||
|---|---|---|---|---|---|---|---|---|---|
| F/(P + U) Mole Ratio | NaOH/P Mole Ratio | Catalyst/P Mole Ratio | |||||||
| Synthesis | Integration b | ||||||||
| PUF1 | 1.4 | 1.37 | 0.40 | 0.04 | 0.96 | 1.17 | 6.75 | 0.31 | 0.47 |
| PUF2 | 1.6 | 1.55 | 1.13 | 1.62 | 5.42 | 0.26 | 0.38 | ||
| PUF3 | 1.8 | 1.70 | 1.24 | 1.80 | 3.31 | 0.12 | 0.24 | ||
| PUF4 | 1.6 | 1.49 | 0.25 | 0.04 | 1.06 | 1.15 | 6.55 | 0.21 | 0.50 |
| PUF5 | 1.55 | 0.40 | 1.13 | 1.62 | 5.42 | 0.26 | 0.38 | ||
| PUF6 | 1.58 | 0.55 | 1.28 | 1.68 | 3.17 | 0.33 | 0.33 | ||
| PUF7 | 1.6 | 1.52 | 0.40 | 0.02 | 1.09 | 1.26 | 6.68 | 0.19 | 0.54 |
| PUF8 | 1.55 | 0.04 | 1.13 | 1.62 | 5.42 | 0.26 | 0.38 | ||
| PUF9 | 1.57 | 0.06 | 1.05 | 1.57 | 3.59 | 0.28 | 0.42 | ||
| PUF0 (Control) | 1.6 | 1.54 | 0.40 | - | 0.92 | 1.06 | 6.86 | 0.16 | 0.59 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pizzi, A. Handbook of Adhesive Technology, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Park, B.D.; Riedl, B.; Bae, H.J.; Kim, Y.S. Differential scanning calorimetry of phenol-formaldehyde (PF) adhesives. J. Wood Chem. Technol. 1999, 19, 265–286. [Google Scholar] [CrossRef]
- Vázquez, G.; López-Suevos, F.; Villar-Garea, A.; González-Alvarez, J.; Antorrena, G. 13C-NMR analysis of phenol-urea-formaldehyde prepolymers and phenol-urea-formaldehyde-tannin adhesives. J. Adhes. Sci. Technol. 2004, 18, 1529–1543. [Google Scholar]
- He, G.B.; Riedl, B. Phenol-urea-formaldehyde cocondensed resol resins: Their synthesis, curing kinetics, and network properties. J. Appl. Polym. Sci. 2003, 41, 1929–1938. [Google Scholar]
- Tomita, B.; Hse, C.Y. Phenol-urea-formaldehyde (PUF) co-condensed wood adhesives. Int. J. Adhes. Adhes. 1998, 18, 69–79. [Google Scholar] [CrossRef]
- Ohyama, M.; Tomita, B.; Hse, C.Y. Curing property and plywood adhesive performance of resol-type phenol-urea-formaldehyde cocondensed resins. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 1995, 49, 87–91. [Google Scholar]
- Zhao, C.; Pizzi, A.; Garnier, S. Fast advancement and hardening acceleration of low condensation alkaline PF resins by ester and copolymerized urea. J. Appl. Polym. Sci. 1999, 74, 359–378. [Google Scholar]
- Fan, D.B.; Li, J.Z.; Chang, J.M. On the structure and cure acceleration of phenol-urea-formaldehyde resins with different catalysts. Eur. Polym. J. 2009, 45, 2849–2857. [Google Scholar] [CrossRef]
- Zhao, C.; Pizzi, A.; Kuhn, A.; Garnier, S. Fast advancement and hardening acceleration of low condensation alkaline phenol-formaldehyde resins by esters and copolymerized urea. II. Esters during resin reaction and effect of guanidine salts. J. Appl. Polym. Sci. 2000, 77, 249–259. [Google Scholar]
- Fan, D.B.; Chang, J.M.; Li, J.Z.; Xia, B.H.; Sang, Z.T. Cure properties and adhesive performances of cure-accelerated phenol-urea-formaldehyde resins. Eur. J. Wood Prod. 2011, 69, 213–220. [Google Scholar]
- Lei, H.; Pizzi, A.; Despres, A.; Pasch, H.; Du, G.B. Esters acceleration mechanisms in phenol-formaldehyde resin adhesives. J. Appl. Polym. Sci. 2006, 100, 3075–3093. [Google Scholar]
- Pizzi, A.; Garcia, R.; Wang, S. On the networking mechanisms of additives-accelerated phenol-formaldehyde polycondensates. J. Appl. Polym. Sci. 1997, 66, 255–266. [Google Scholar] [CrossRef]
- Fraser, D.A.; Hall, R.W.; Raum, A.L.J. Preparation of ‘high-ortho’ novolak resins. I. Metal ion catalysis and orientation effect. J. Appl. Chem. 1957, 7, 676–689. [Google Scholar]
- Huang, J.Y.; Xu, M.Q.; Ge, Q.; Lin, M.H.; Lin, Q.; Chen, Y.H.; Chu, J.C.; Dai, L.Z.; Zou, Y.S. Controlled synthesis of high-ortho-substitution phenol-formaldehyde resins. J. Appl. Polym. Sci. 2005, 97, 652–658. [Google Scholar] [CrossRef]
- Fan, D.B.; Chu, F.X.; Qin, T.F.; Li, J.Z. Effect of synthesis conditions on the structure and curing characteristics of high-urea content PUF resin. J. Adhes. 2011, 87, 1191–1203. [Google Scholar] [CrossRef]
- He, G.B.; Yan, N. 13C-NMR study on structure, composition and curing behavior of phenol-urea-formaldehyde resole resins. Polymer 2004, 45, 6813–6822. [Google Scholar] [CrossRef]
- Holopained, T.; Alvila, L.; Rainio, J.; Pakkanen, T.T. Phenol-formaldehyde resol resins studied by 13C-NMR spectroscopy, gel permeation chromatography, and differential scanning calorimetry. J. Appl. Polym. Sci. 1997, 66, 1183–1193. [Google Scholar] [CrossRef]
- Luukko, P.; Alvila, L.; Holopained, T.; Rainio, J.; Pakkanen, T.T. Effect of alkalinity on the structure of phenol-formaldehyde resol resins. J. Appl. Polym. Sci. 2001, 82, 258–262. [Google Scholar] [CrossRef]
- Philbrook, A.; Earnshaw, S.; Easton, C.J.; Keniry, M.A.; Latter, M.J. Co-polymerization analysis of thermosetting resins using 1H-15N-13C triple resonance NMR spectroscopy. J. Appl. Polym. Sci. 2013, 128, 3375–3381. [Google Scholar] [CrossRef]
- Poljanšek, I.; Urška, Š.; Krajnc, M. Characterization of phenol-urea-formaldehyde resin by inline FTIR spectroscopy. J. Appl. Polym. Sci. 2006, 99, 2016–2028. [Google Scholar]
- Du, G.B.; Lei, H.; Pizzi, A.; Pasch, H. Synthesis-Structure-Performance relationship of cocondensed phenol-urea-formaldehyde resins by MALDI-TOF and 13C-NMR. J. Appl. Polym. Sci. 2008, 110, 1182–1194. [Google Scholar] [CrossRef]
- Angelatos, A.S.; Burgar, M.I.; Dunlop, N.; Separovic, F. NMR structural elucidation of amino resins. J. Appl. Polym. Sci. 2004, 91, 3504–3512. [Google Scholar] [CrossRef]
- Fan, D.B.; Chang, J.M.; Li, J.Z.; Mao, A.; Zhang, L.T. 13C-NMR study on the structure of phenol-urea-formaldehyde resins prepared by methylolureas and phenol. J. Appl. Polym. Sci. 2009, 112, 2195–2202. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. I. Phenolic chelates. J. Appl. Polym. Sci. 1979, 24, 1247–1255. [Google Scholar]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. J. Appl. Polym. Sci. 1979, 24, 1257–1268. [Google Scholar]
- Pizzi, A.; Stephanou, A.; Antunes, I.; Debeer, G. Alkaline PF resins linear extension by urea condensation with hydroxybenzylalcohol groups. J. Appl. Polym. Sci. 1993, 50, 2201–2207. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fan, D.-B.; Li, G.-Y.; Qin, T.-F.; Chu, F.-X. Synthesis and Structure Characterization of Phenol-Urea-Formaldehyde Resins in the Presence of Magnesium Oxide as Catalyst. Polymers 2014, 6, 2221-2231. https://doi.org/10.3390/polym6082221
Fan D-B, Li G-Y, Qin T-F, Chu F-X. Synthesis and Structure Characterization of Phenol-Urea-Formaldehyde Resins in the Presence of Magnesium Oxide as Catalyst. Polymers. 2014; 6(8):2221-2231. https://doi.org/10.3390/polym6082221
Chicago/Turabian StyleFan, Dong-Bin, Gai-Yun Li, Te-Fu Qin, and Fu-Xiang Chu. 2014. "Synthesis and Structure Characterization of Phenol-Urea-Formaldehyde Resins in the Presence of Magnesium Oxide as Catalyst" Polymers 6, no. 8: 2221-2231. https://doi.org/10.3390/polym6082221
APA StyleFan, D. -B., Li, G. -Y., Qin, T. -F., & Chu, F. -X. (2014). Synthesis and Structure Characterization of Phenol-Urea-Formaldehyde Resins in the Presence of Magnesium Oxide as Catalyst. Polymers, 6(8), 2221-2231. https://doi.org/10.3390/polym6082221