Intraoral Temperature Triggered Shape-Memory Effect and Sealing Capability of A Transpolyisoprene-Based Polymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Shape-Memory Polymer Preparation
| Code | TPI:100-S:0.5 | TPI:85-S:0.5 | TPI:70-S:0.5 | TPI:50-S:0.5 | TPI:100-S:0.75 | TPI:100-S:1.0 | TPI:100-S:1.25 |
|---|---|---|---|---|---|---|---|
| Trans-1,4-polyisoprene | 100 | 85 | 70 | 50 | 100 | 100 | 100 |
| Cis-1,4-polyisoprene | 0 | 15 | 30 | 50 | 0 | 0 | 0 |
| Zinc oxide | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Stearic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sulfur | 0.5 | 0.5 | 0.5 | 0.5 | 0.75 | 1.0 | 1.25 |
| Dicumyl peroxide | 3 | 3 | 3 | 3 | 4.5 | 6.0 | 7.5 |

2.2. Measurement of Shape Recovery Ratio at a Fixed Constant Temperature for the Standard Material
2.3. Measurement of Shape Recovery under A Constant Rate Rising Temperature
2.4. Measurement of Recovery Stress under a Constant Rate Rising Temperature
2.5. Measurement of Relaxation Modulus at a Fixed Constant Temperature
2.6. Measurement of the Shape Recovery Ratio at a Fixed Constant Temperature of 37 °C
2.7. Sealing Ability Test Using Glass Tubing



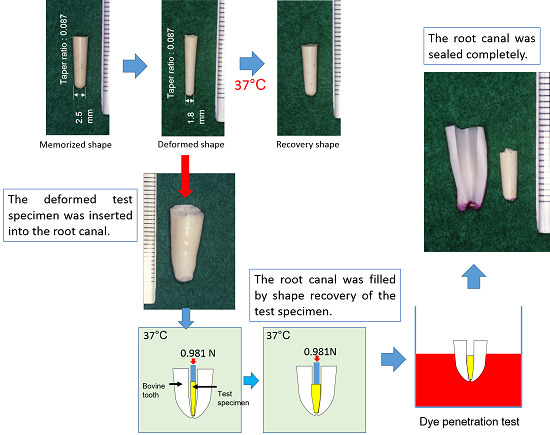
2.8. Sealing Ability Test Using Bovine Incisor



3. Results
3.1. Shape Recovery Ratio under A Fixed Constant Temperature for the Standard Material

3.2. Shape Recovery under a Constant Rate Rising Temperature

3.3. Recovery Stress under a Constant Rate Rising Temperature

3.4. Relaxation Modulus under a Fixed Constant Temperature

3.5. Measurement of the Shape Recovery Ratio at 37 °C

3.6. Sealing Ability using Glass Tubing

3.7. Sealing Ability using a Bovine Incisor


4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Timpawat, S.; Amornchat, C.; Trisuwan, W.R. Bacterial coronal leakage after obturation with three root canal sealers. J. Endod. 2001, 27, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.U.; Kececi, A.D.; Berri, S. Evaluation of the sealing ability of gutta-percha and thermoplastic synthetic polymer-based systems along the root canals through the glucose penetration model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Brothman, P. A comparative study of the vertical and lateral condensation of gutta-percha. J. Endod. 1981, 7, 27–30. [Google Scholar] [CrossRef]
- Mann, S.R.; McWalter, G.M. Evaluation of apical seal and placement control in straight and curved canals obturated by laterally condensed and thermoplasticized gutta-percha. J. Endod. 1987, 13, 10–17. [Google Scholar] [CrossRef]
- Canalda-Sahli, C.; Berástegui-Jimeno, E.; Brau-Aguadé, E. Apical sealing using two thermoplasticized gutta-percha techniques compared with lateral condensation. J. Endod. 1997, 23, 636–638. [Google Scholar] [CrossRef]
- Sobhnamayan, F.; Sahebi, S.; Moazami, F.; Borhanhaghighi, M. Comparison of apical sealing ability of lateral condensation technique in room temperature and body-stimulated temperature (An in vitro study). J. Dent. Shiraz Univ. Med. Scien. 2013, 14, 25–30. [Google Scholar]
- Allison, D.A.; Michelich, R.J.; Walton, R.E. The influence of master cone adaptation on the quality of the apical seal. J. Endod. 1981, 7, 61–65. [Google Scholar] [CrossRef]
- Johnson, W.B.; Okla, T. A new gutta-percha tequnique. J. Endod. 1978, 4, 184–188. [Google Scholar] [CrossRef]
- Moreno, A.; Mexico, M. Thermomechanically softened gutta-percha root canal filling. J. Endod. 1977, 3, 186–188. [Google Scholar] [CrossRef]
- Schilder, H. Filling root canals in three dimensions. Dent. Clin. North Am. 1967, 11, 723–744. [Google Scholar] [CrossRef] [PubMed]
- Yee, F.S.; Marlin, J.; Kradow, A.A.; Gron, P. Three-dimensional obturation of the root canal using injection-molded, thermoplasticized dental gutta-percha. J. Endod. 1977, 3, 168–174. [Google Scholar] [CrossRef]
- Clinton, K.; Himel, V.T. Comparison of a warm gutta-percha obturation technique and lateral condensation. J. Endod. 2001, 27, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.N.; Kimbrough, W.F.; Anderson, R.W. A comparison of thermoplastic obturation techniques: Adaptation to the canal walls. J. Endod. 1997, 23, 703–706. [Google Scholar] [CrossRef]
- Lacombe, J.S.; Campbell, A.D.; Hicks, M.L.; Pelleu, G.B. A comparison of the apical seal producted by two thermoplasticized injectable gutta-percha techniques. J. Endod. 1988, 14, 445–450. [Google Scholar] [CrossRef]
- Hata, G.-I.; Kawazoe, S.; Toda, T.; Weine, F.S. Sealing ability of thermal with and without sealer. J. Endod. 1992, 18, 322–326. [Google Scholar] [CrossRef]
- Skinner, R.L.; Himel, V.T. The sealing ability of injection–molded thermoplasticized gutta-percha with and without the use of sealers. J. Endod. 1987, 13, 315–317. [Google Scholar] [CrossRef]
- Evans, J.T.; Simon, J.H.S. Evaluation of the apical seal producted by injected thermoplasticized gutta-percha in the absence of smear layer and root canal sealer. J. Endod. 1986, 12, 101–107. [Google Scholar] [CrossRef]
- Schilder, H.; Goodman, A.; Aldrich, W. The thermomechanical properties of gutta-percha: Part V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 285–296. [Google Scholar] [CrossRef]
- Lee, C.Q.; Chang, Y.; Cobb, C.M.; Robinson, S.; Hellmuth, E.M. Dimensional stability of thermosensitive gutta-percha. J. Enod. 1997, 23, 579–582. [Google Scholar] [CrossRef]
- Tsukada, G.; Tanaka, T.; Torii, M.; Inoue, K. Shear modulus and thermal properties of gutta percha for root canal filling. J. Oral. Rehabil. 2004, 31, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Ye, L.; Tan, H.; Zbou, X. Outcome of root canal obturation by warm gutta-percha versus cold lateral condensation: A meta-analysis. J. Endod. 2007, 33, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Dewani, N.; Hayes, S.J.; Dummer, P.M.H. Comparison of laterally condensed and low-temperature thermoplasticized gutta-percha root fillings. J. Endod. 2000, 26, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.M.; Sandrik, J.L.; Heuer, M.A.; Rapp, G.W. Composition and mechanical properties of gutta-percha endodontic points. J. Dent. Res. 1975, 54, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Marciano, J.; Michailesco, P.M. Dental Gutta-percha: Chemical composition, X-ray identification, enthalpic studies, and clinical implication. J. Endod. 1989, 15, 149–153. [Google Scholar] [CrossRef]
- Gurgel-Filho, E.D.; Feitosa, J.P.A.; Teixeira, F.B.; de Paula, R.C.M.; Silva, J.B.A.; Souza-Filho, F.J. Chemical and X-ray analyses of five brands of dental gutta-percha cone. Int. Endod. J. 2003, 36, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Spangberg, L.; Langeland, K.; Conn, F. Biologic effects of dental materials: 1. Toxicity of root canal filling materials on HeLa cells in vitro. Oral Surg. 1973, 35, 402–414. [Google Scholar] [CrossRef]
- Pascon, E.A.; Spångberg, L.S.W. In vitro cytotoxicity of root canal filling materials: 1. Gutta-percha. J. Endod. 1990, 16, 429–433. [Google Scholar] [CrossRef]
- Goodman, A.; Schilder, H.; Aldrich, W. The thermomechanical properties of gutta-percha: 2. The history and molecular chemistry of gutta-percha. Oral Surg. 1974, 37, 954–961. [Google Scholar] [CrossRef]
- Davies, C.K.L.; Long, O.E. Morphology of trans-1, 4-polyisoprene crystallized in thin films. J. Mater. Sci. 1977, 12, 2165–2183. [Google Scholar] [CrossRef]
- Yoshikawa, R. Transpolyiosprene. J. Soc. Rubber Ind. 1984, 57, 723–727. [Google Scholar] [CrossRef]
- Ishii, M. Shape memory plastics: Polyisoprene based plastics. Plastic Age 1989, 35, 158–164. [Google Scholar]
- Tsukada, G.; Tokuda, M.; Torii, M. Temperature triggered shape memory effect of transpolyisoprene-based polymer. J. Endod. 2014, 40, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Marer. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Small, W.; Singhal, P.; Wilson, T.S.; Maitland, D.J. Biomedical applications of thermally activated shape memory polymers. J. Mater. Chem. 2010, 20, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape-memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Tong, L.; Lv, H.; Liang, W. Shape memory and thermo-mechanical properties of shape memory polymer/carbon fiber composites. Compos. Part A 2015, 76, 162–171. [Google Scholar] [CrossRef]
- Ebara, M. Shape-memory surfaces for cell mechanobiology. Sci. Technol. Adv. Mater. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Dow, P.R.; Ingle, J.I. Isotope determination of root canal failure. Oral Surg. Oral Med. Oral Pathol. 1955, 8, 1100–1104. [Google Scholar] [CrossRef]
- Kersten, H.W.; Moorer, W.R. Particles and molecules in endodontic leakage. Int. Endod. J. 1989, 22, 118–124. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukada, G.; Kato, R.; Tokuda, M.; Nishitani, Y. Intraoral Temperature Triggered Shape-Memory Effect and Sealing Capability of A Transpolyisoprene-Based Polymer. Polymers 2015, 7, 2259-2275. https://doi.org/10.3390/polym7111512
Tsukada G, Kato R, Tokuda M, Nishitani Y. Intraoral Temperature Triggered Shape-Memory Effect and Sealing Capability of A Transpolyisoprene-Based Polymer. Polymers. 2015; 7(11):2259-2275. https://doi.org/10.3390/polym7111512
Chicago/Turabian StyleTsukada, Gakuji, Ryuzo Kato, Masayuki Tokuda, and Yoshihiro Nishitani. 2015. "Intraoral Temperature Triggered Shape-Memory Effect and Sealing Capability of A Transpolyisoprene-Based Polymer" Polymers 7, no. 11: 2259-2275. https://doi.org/10.3390/polym7111512
APA StyleTsukada, G., Kato, R., Tokuda, M., & Nishitani, Y. (2015). Intraoral Temperature Triggered Shape-Memory Effect and Sealing Capability of A Transpolyisoprene-Based Polymer. Polymers, 7(11), 2259-2275. https://doi.org/10.3390/polym7111512