The world consumption of polyolefins and, more specifically polypropylene (PP), represented more than 50 million tons in 2009 and is estimated to have had an average annual growth rate of 4% for the period from 2010 to 2020 [
1]. In contrast to other polymers like polystyrene or polyvinyl chloride, PP is widely used in producing engineering plastics. Its price varies according to three elements,
i.e., its alkylation value, its netback depending on the parity between high-density polyethylene and resins such as PS. Polypropylene has replaced other polymers in the production of a wide range of fibrous materials in household textiles (carpet backing and yarns, upholstery fabrics, rugs, and others), in health and medicine for care materials or disposable diapers, in protective clothing, in geo and/or agrotextiles, nonwovens, and others. Thus, the production of PP fibers represented 10% of the volume of fibers manufactured in 2007 globally. Even though the consumption of polypropylene textile materials in Europe decreased about 4.8% from 2008 to 2009, polyolefin fibers represented 44.3% of the consumption of man-made fiber in 2010 mainly in spunbond and meltblown (31.2%), tapes and slit films (21.8%), multifilaments (19.1%) and staple fibers (20.7%). The needs of consumer protection, coupled with the new regulations and environmental concerns, require the development of more eco-friendly flame-retardant treatments for PP fibers. Improvements in flame retardancy of the fiber-forming polymers, including PP, have become increasingly important in recent years, to comply with the safety requirements of textile products for automotive and home furnishing [
2]. Flammability of PP fibers represents one of the main drawbacks for the use of this polymer, due to its aliphatic hydrocarbon structure. This combustible material ignites spontaneously and can be ignited at about 360 and 345 °C, respectively. Since the burning leads to the formation of droplets spreading the fire, the reduction of flammability of PP is needed for safety consideration. Furthermore, polypropylene has a high heat of combustion, about 46.4 kJ·g
−1, and no char-forming character with a limiting oxygen index (LOI) of about 18%. Its ignition time is relatively low compared with some other thermoplastics, and even if it may be classified as a polymer with low smoke emission, its high peak of heat release and its rapid production of smoke constitute a hazard in real fire situations. To prevent the burning and to enhance the fire retardant (FR) properties of textile structures, various solutions have been studied. One of them introduces a single additive or a mixture of additives to provide a synergistic effect and/or to reduce cost, into the polymer by melt mixing processes or onto the surface by finishing treatments. One way recognized to develop effective intumescent systems is the use of metal hydroxides such as magnesium and aluminum. Nevertheless, a high amount of additive is required, about 60 wt% and 25 wt% to 30 wt% for single or mixture of FR, respectively [
3]. This loading content is not suitable to manufacture fiber with conventional textile processes, since the addition of such a high content of additive into the fibers decreases their mechanical properties, which limits their use in conventional spinning textile process. The use of high loadings of additives can be only achieved by surface coating processes, which can impair properties of the substrate such as softness, drapability,
etc. Typically, the amount of additives within a fiber should not exceed 10 wt% and more generally should be restricted to 5 wt% or 6 wt% for a flame retardant polypropylene fiber produced by melt-spinning. Furthermore, the additives have to be thermally stable during the manufacturing process and compatible with the polymer matrix. They should have particle sizes that will not affect the fiber spinning process, they should not change the color of the final substrate, and should have no leaching properties. Furthermore, they should retain their flame retardant properties after textile processing. They should function at a lower temperature than the degradation temperature of the PP and should reduce the toxicity of gases and smoke produced during burning to an acceptable level.
Bourbigot
et al. reviewed several solutions for flame retarding textile structures [
4]. For the PP fabrics, these solutions were mainly based on nano-additives and carbon nanotubes, but their efficiencies were limited, even if good results were obtained by cone calorimeter study. Furthermore, the use of carbon nanotubes leads to a black fiber. Zhang
et al. obtained an increase in FR performance by adding 6 wt% of a mixture of clay (Cloisite
® 30B, Southern Clay Products, Inc., Gonzales, TX, USA), ammonium polyphosphate and a sterically-hindered amine [
2]. Despite these results, the low thermal stability and the relatively high particle size of ammonium polyphosphate restrict its use for spinning. One current solution is the use of halogenated products in the core of the fibers. For example, the product @2spin PP201™ (Devan Chemicals, Ronse, Belgium) achieves an M1 rating for a PP nonwoven with only 3 wt% of additives. The last decade has witnessed a desire to reduce the impact of flame retardants generally on the eco-system and much research has moved towards halogen-free systems and/or their combination with lower amounts of halogen to allow substantial decreases in the halogenated substances present to occur [
5]. Another possibility is the synthesis or the formulation of multifunctional flame retardants, such as Flamestab
® Nor 116 (BASF, Basel, Switzerland) which is mainly used for polypropylene fibers or films [
6]. In this context, Roth and coworkers have developed new nitrogen based flame retardant compounds that exhibit self-extinguishing properties for polypropylene compounds [
5,
7,
8,
9].
Recently announced mixtures of phosphinates and other phosphorus compounds [
10,
11,
12,
13,
14] have enabled Devan to develop for PP a novel flame retardant masterbatch. The aim of this article is to present the main results obtained by using this new mixture of phosphinates (MPh), in combination with classical nitrogen synergist agent, melamine cyanurate (MC), in different polypropylene textile structures. All the studies were made with a total amount of 6 wt% of FR additives. In the first part, the impact of two kinds of textile structures with a chosen ratio of the both FR additives in the combination was investigated. In the second part, the influence of the ratio between the melamine cyanurate (MC) and a mixture of phosphinate (MPh) for a nonwoven support was investigated. The influence of FR additives on the thermal properties and stability of the PP fibers were investigated by DSC (Differential scanning calorimetry) and TGA (Thermogravimetric analysis), and the estimation of fire behavior of the textile fabrics by cone calorimeter and limiting oxygen index (LOI) measurements are discussed. Furthermore, two other standard tests for industrial applications,
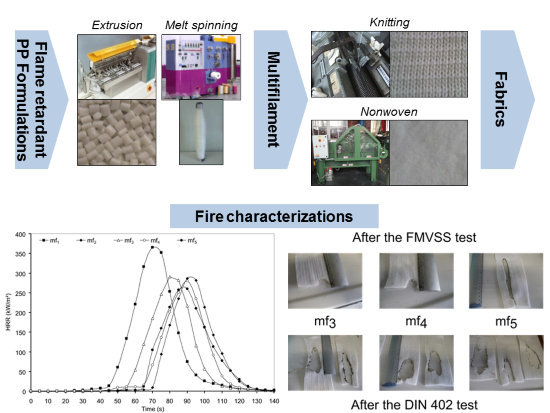
i.e., DIN 4102-1 part B [
15] and FMVSS 302 [
16] are used to determine the potential of the use of these new additives in textiles for automotive and building applications.