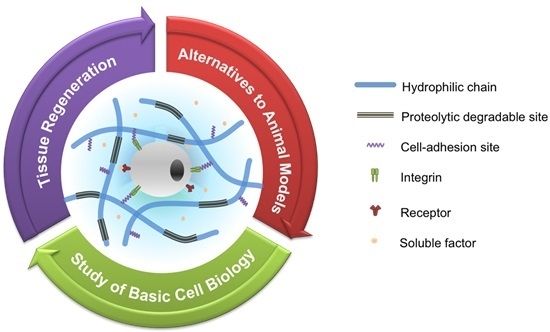
Engineered Polymeric Hydrogels for 3D Tissue Models
Abstract
:1. Introduction
2. Polymeric Hydrogel Matrices

2.1. Natural Hydrogels
2.2. Synthetic Hydrogels
2.3. Semi-Synthetic Hydrogels
3. Engineered 3D Tissue Models
| Type of polymer (polymer backbone) | Crosslinking method | Cell source | Engineered 3D tissue models | Applications | Reference |
|---|---|---|---|---|---|
| Natural (collagen) | Thermogelation | NHEKs, NHDFS, SCC-12B and SCC13 | Skin tissues (in vitro models for normal skin and human cutaneous SCC) | - Studying the molecular mechanism of carcinoma progression; - Assess the effect of EGFR activation and inhibition on SCC progression | [32] |
| Natural (collagen) | Thermogelation | ADSCs | Skin tissues (tissue-engineered dermo-epidermal skin grafts) | - Evaluating prevascularized skin graft | [33] |
| Synthetic (PEG) | Chemical crosslinking (click-chemistry) | ECs and mural cells (MSCS, SMCs, HDFs) | In vitro angiogenesis models | - Studying the regulation of heterocellular communication | [34] |
| Semi-synthetic (gelatin) | Chemical crosslinking (laccase-mediated crosslinking reaction) | ECFCs | Vascular tissues | - Creating 3D vasculatures; - Studying basic cell biology for the hypoxia effect on vascular morphogenesis | [22] |
| Semi-synthetic (HA/gelatin) | Chemical crosslinking (photo-crosslinking reaction) | GBM | Tumor models (brain tumor models) | - Studying the effect of spatial gradation on brain tumor cells | [35] |
| Semi-synthetic (HA) | Chemical crosslinking (Michael-type addition reaction) | HT1080 and ECFCs | Tumor models (tumor angiogenesis models) | - Investigating the effect of matrix stiffness and oxygen tension on vascular cell invasion | [36] |
| Semi-synthetic (HA) | Chemical crosslinking (click-reaction) | MCF-7, T-47D, SK-MEL-28 and MDA-MB-231 | Tumor models (tumor invasion models) | - Studying the effect of matric stiffness and cell adhesion ligand density on cancer cell invasion | [31] |
| Semi-synthetic (PEG/heparin) | Chemical crosslinking (maleimide-mediated crosslinking reaction) | HUVECs, MSCs, MCF-7, MDA-MB-231, LNCaP, PC3 | Tumor models (tumor angiogenesis models) | - Tri-culture systems to investigate the effect of cell components on tumor angiogenesis and drug resistance | [37] |
| Semi-synthetic (PEG) | Chemical crosslinking (photo-crosslinking reaction) | Hepatocytes | Liver models (hepatic tissue models) | - Investigating the effect of hepatocyte density on the in vitro function of hepatic tissues; - Liver tissue regeneration | [38] |
| Semi-synthetic (PEG) | Chemical crosslinking (photo-crosslinking reaction) | Human embryonic stem cell-derived pancreatic precursor cell aggregates | Pancreatic islet models | - Studying the effect of collage type I on islet aggregate formation and their viability within the microenvironment | [39] |
3.1. Vascular Tissues

3.2. Skin Tissues
3.3. Tumor Models
3.4. Other Tissue Models
4. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| ECMs | extracellular matrices |
| HA | hyaluronic acid |
| PVA | poly(vinyl alcohol) |
| PNIPAAm | poly(N-isopropylacrylamide) |
| PEO–PPO–PEO | poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) |
| MMP | matrix metalloproteinase |
| GAG | glycosaminoglycan |
| HIFs | hypoxia-inducible factors |
| FA | ferulic acid |
| Gtn | gelatin |
| GFs | growth factors |
| VEFG | vascular endothelial growth factor |
| bFGF | basic fibroblast growth factor |
| SDF-1α | stromal-derived growth factor-1α |
| ADSCs | adipose-derived stem cells |
| ECs | endothelial cells |
| EPCs | endothelial progenitor cells |
| ECFCs | endothelial colony-forming cells |
| EGFR | epidermal growth factor receptor |
| GBM | glioblastoma multiforme |
| HDFs | human dermal fibroblasts |
| SK-MEL-28 | skin melanoma cell line |
| HT1080 | human fibrosarcomas |
| MCF-7 | human breast adenocarcinoma cell line |
| MDA-MB-23 | human breast adenocarcinoma cell line |
| MSCs | mesenchymal stem cells |
| NHDFs | primary normal human dermal fibroblasts |
| NHEKs | primary normal human epidermal keratinocytes |
| PEG | poly(ethylene glycol) |
| SCC | squamous cell carcinoma |
| SMCs | smooth muscle cells |
| T-47D | human ductal breast epithelial tumor cell line |
References
- Angelova, N.; Hunkeler, D. Rationalizing the design of polymeric biomaterials. Trends Biotechnol. 1999, 17, 409–421. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Polymeric biomaterials in tissue engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Baudis, S.; Nehl, F.; Ligon, S.C.; Nigisch, A.; Bergmeister, H.; Bernhard, D.; Stampfl, J.; Liska, R. Elastomeric degradable biomaterials by photopolymerization-based CAD–CAM for vascular tissue engineering. Biomed. Mater. 2011, 6, 055003. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Li, X.; Chung, U.I.; Sakai, T. Design of hydrogels for biomedical applications. Adv. Healthc. Mater. 2015, 4, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, N.; Kumar, N.; Kumar, M.N. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Curcio, M.; Cirillo, G.; Spataro, T.; Vittorio, O.; Picci, N.; Hampel, S.; Iemma, F.; Nicoletta, F.P. Recent advances in the synthesis and biomedical applications of nanocomposite hydrogels. Pharmaceutics 2015, 7, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D. Cell Biology of Extracellular Matrix, 2nd ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Francia, G.; Kerbel, R.S. Raising the bar for cancer therapy models. Nat Biotechnol. 2010, 28, 561–562. [Google Scholar] [CrossRef] [PubMed]
- DeForest, C.A.; Anseth, K.S. Advances in bioactive hydrogels to probe and direct cell fate. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Dewi, R.E.; Heilshorn, S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015, 25, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Son, J.Y.; Choi, J.H.; Kim, I.G.; Lee, Y.; Lee, J.Y.; Park, K.D. Macro/Nano-gel composite as an injectable and bioactive bulking material for the treatment of urinary incontinence. Biomacromolecules 2014, 15, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Lu, H.D.; Charati, M.B.; Kim, I.L.; Burdick, J.A. Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials 2012, 33, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Arunkumar, R.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Benton, J.A.; Anseth, K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 2010, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Gerecht, S. Hypoxia-inducible hydrogels. Nat. commun. 2014, 5, 4075. [Google Scholar] [CrossRef] [PubMed]
- Deforest, C.A.; Sims, E.A.; Anseth, K.S. Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chem. Mater. Publ. Am. Chem. Soc. 2010, 22, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.M.; Kyburz, K.A.; Anseth, K.S. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad.Sci. USA 2015, 112, E3757–E3764. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, D.; Srouji, S.; Shapira-Schweitzer, K.; Kossover, O.; Ivanir, E.; Kuhn, G.; Müller, R.; Seliktar, D.; Livne, E. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials 2013, 34, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Blatchley, M.; Park, K.M.; Gerecht, S. Designer hydrogels for precision control of oxygen tension and mechanical properties. J. Mater. Chem. B 2015, 3, 7939–7949. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.J.; Martino, M.M.; de Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2013, 2, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Yoon, H.Y.; Koo, H.; Ko, S.-H.; Shim, J.-S.; Lee, J.-H.; Kim, K.; Kwon, I.C.; Kim, D.-D. Self-assembled nanoparticles based on hyaluronic acid-ceramide (HA-CE) and Pluronic® for tumor-targeted delivery of docetaxel. Biomaterials 2011, 32, 7181–7190. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Anandakumaran, P.N.; Owen, S.C.; Shoichet, M.S. Tuning the microenvironment: Click-crosslinked hyaluronic acid-based hydrogels provide a platform for studying breast cancer cell invasion. Adv. Funct. Mater. 2015. [Google Scholar] [CrossRef]
- Commandeur, S.; van Drongelen, V.; de Gruijl, F.R.; El Ghalbzouri, A. Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma. Cancer Sci. 2012, 103, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Bottcher-Haberzeth, S.; Biedermann, T.; Schiestl, C.; Reichmann, E.; Meuli, M. Analysis of blood and lymph vascularization patterns in tissue-engineered human dermo-epidermal skin analogs of different pigmentation. Pediatr. Surg. Int. 2014, 30, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, K.; Tsurkan, M.V.; Freudenberg, U.; Werner, C. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Sci. Rep. 2014, 4, 4414. [Google Scholar] [CrossRef] [PubMed]
- Pedron, S.; Becka, E.; Harley, B.A. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv. Mater. 2015, 27, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-I.; Abaci, H.E.; Krupski, Y.; Weng, L.-C.; Burdick, J.A.; Gerecht, S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater. Sci. 2014, 2, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Miller, J.S.; Blakely, B.L.; Chen, C.S.; Bhatia, S.N. Degradable hydrogels derived from PEG-diacrylamide for hepatic tissue engineering. J. Biomed.Mater. Res. A 2015, 103, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Amer, L.D.; Holtzinger, A.; Keller, G.; Mahoney, M.J.; Bryant, S.J. Enzymatically degradable poly(ethylene glycol) hydrogels for the 3D culture and release of human embryonic stem cell derived pancreatic precursor cell aggregates. Acta Biomater. 2015, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Au, P.; Tam, J.; Duda, D.G.; Fukumura, D. Engineering vascularized tissue. Nat. Biotechnol. 2005, 23, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Gerecht, S. Harnessing developmental processes for vascular engineering and regeneration. Development 2014, 141, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Mazzone, M.; Carmeliet, P. Antiangiogenic therapy, hypoxia, and metastasis: Risky liaisons, or not? Nat. Rev.Clin. oncol. 2011, 8, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2012, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Guven, S.; Biedermann, T.; Luginbuhl, J.; Bottcher-Haberzeth, S.; Meuli-Simmen, C.; Meuli, M.; Martin, I.; Scherberich, A.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef] [PubMed]
- Song, H.H.; Park, K.M.; Gerecht, S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv. Drug Deliv. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Wang, L.; Han, S.; Pingguan-Murphy, B.; Zhang, X.; Xu, F. Engineering of microscale three-dimensional pancreatic islet models in vitro and their biomedical applications. Crit. Rev. Biotechnol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, V.; Trietsch, S.J.; Joore, J.; Vulto, P.; Hankemeier, T. Microfluidic 3D cell culture: from tools to tissue models. Curr. opin. Biotechnol. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, K.M. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23. https://doi.org/10.3390/polym8010023
Park S, Park KM. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers. 2016; 8(1):23. https://doi.org/10.3390/polym8010023
Chicago/Turabian StylePark, Sujin, and Kyung Min Park. 2016. "Engineered Polymeric Hydrogels for 3D Tissue Models" Polymers 8, no. 1: 23. https://doi.org/10.3390/polym8010023
APA StylePark, S., & Park, K. M. (2016). Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers, 8(1), 23. https://doi.org/10.3390/polym8010023