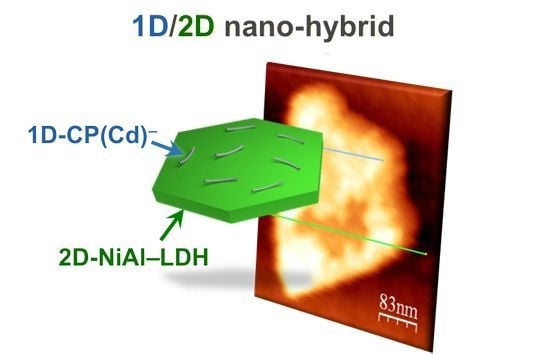
Self-Assembly of 1D/2D Hybrid Nanostructures Consisting of a Cd(II) Coordination Polymer and NiAl-Layered Double Hydroxides
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Synthesis of NiAl–LDH
2.1.2. Exfoliation of NiAl-NO3 LDH
2.1.3. Preparation of the Na2n[Cd(6-MP2−)2]n
2.1.4. Synthesis of 1D/2D hybrid Material
2.2. Methods
3. Results and Discussion




| Sample | Cd3d | S2p | N1s | Al2p | Ni2p3/2 | C1s |
|---|---|---|---|---|---|---|
| 1D-CP(Cd) | 404.5 | 161.8 | 398.4 (76) * 400.2 (24) * | – | – | 284.8 |
| NiAl–LDH | – | – | – | 74.2 | 856.2 | 288.9 |
| 1D/2D hybrid | 404.8 | 161.8 | 398.3 | 74.5 | 856.9 | 284.8 |




4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanchez, C.; Shea, K.J.; Kitagawa, S. Recent progress in hybrid materials science. Chem. Soc. Rev. 2011, 40, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, P.; Sanchez, C. Functional Hybrid Materials; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Ogawa, M. Hybrid and biohybrid silicate based materials: Molecular vs. block-assembling bottom-up processes. Chem. Soc. Rev. 2011, 40, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Abellán, G.; Martí-Gastaldo, C.; Ribera, A.; Coronado, E. Acc. Chem. Res. 2015, 48, 1601–1611.
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Evans, D. Layered Double Hydroxides; Springer: Berlin, Germany, 2006. [Google Scholar]
- Rives, V. Layered Double Hydroxides: Present and Future; Nova Science Publishers, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ma, R.Z.; Sasaki, T. Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Adv. Mater. 2010, 22, 5082–5104. [Google Scholar] [CrossRef] [PubMed]
- Abellan, G.; Coronado, E.; Marti-Gastaldo, C.; Pinilla-Cienfuegos, E.; Ribera, A. Hexagonal nanosheets from the exfoliation of Ni2+–Fe3+ LDHs: A route towards layered multifunctional materials. J. Mater. Chem. 2010, 20, 7451–7455. [Google Scholar] [CrossRef]
- Mas-Balleste, R.; Gomez-Navarro, C.; Gomez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Marti-Gastaldo, C.; Navarro-Moratalla, E.; Ribera, A.; Blundell, S.J.; Baker, P.J. Coexistence of superconductivity and magnetism by chemical design. Nat. Chem. 2010, 2, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Zhu, G.; Liu, Z.H.; Yang, Z.P.; Wang, Z.L. Fabrication of a hybrid graphene/layered double hydroxide material. Carbon 2010, 48, 4391–4396. [Google Scholar] [CrossRef]
- Latorre-Sanchez, M.; Atienzar, P.; Abellan, G.; Puche, M.; Fornes, V.; Ribera, A.; Garcia, H. The synthesis of a hybrid graphene-nickel/manganese mixed oxide and its performance in lithium-ion batteries. Carbon 2012, 50, 518–525. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides—Properties, synthesis, and applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D. Coordination Polymers: Design, Analysis and Applications; RSC Publishing: London, UK, 2009; Volume 7. [Google Scholar]
- Foo, M.L.; Matsuda, R.; Kitagawa, S. Functional hybrid porous coordination polymers. Chem. Mater. 2014, 26, 310–322. [Google Scholar] [CrossRef]
- Garcia-Couceiro, U.; Olea, D.; Castillo, O.; Luque, A.; Roman, P.; de Pablo, P.J.; Gomez-Herrero, J.; Zamora, F. Scanning probe microscopy characterization of single chains based on a one-dimensional oxalato-bridged manganese(II) complex with 4-aminotriazole. Inorg. Chem. 2005, 44, 8343–8348. [Google Scholar] [CrossRef] [PubMed]
- Olea, D.; Alexandre, S.S.; Amo-Ochoa, P.; Guijarro, A.; de Jesus, F.; Soler, J.M.; de Pablo, P.J.; Zamora, F.; Gomez-Herrero, J. From coordination polymer macrocrystals to nanometric individual chains. Adv. Mater. 2005, 17, 1761–1765. [Google Scholar] [CrossRef]
- Olea, D.; Garcia-Couceiro, U.; Castillo, O.; Gomez-Herrero, J.; Zamora, F. Nanoprocessability of a one-dimensional oxalato-bridged cobalt(II) complex with 1,2,4-triazole. Inorg. Chim. Acta 2007, 360, 48–54. [Google Scholar] [CrossRef]
- Olea, D.; Gonzalez-Prieto, R.; Priego, J.L.; Barral, M.C.; de Pablo, P.J.; Torres, M.R.; Gomez-Herrero, J.; Jimenez-Aparicio, R.; Zamora, F. Mmx polymer chains on surfaces. Chem. Commun. 2007, 2007, 1591–1593. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Welte, L.; González-Prieto, R.; Sanz Miguel, P.J.; Gómez-García, C.J.; Mateo-Martí, E.; Delgado, S.; Gómez-Herrero, J.; Zamora, F. Single layers of a multifunctional laminar Cu(I,II) coordination polymer. Chem. Commun. 2010, 46, 3262–3264. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Hermosa, C.; Castillo, O.; Berlanga, I.; Gomez-Garcia, C.J.; Mateo-Marti, E.; Martinez, J.I.; Flores, F.; Gomez-Navarro, C.; Gomez-Herrero, J.; et al. Solvent-induced delamination of a multifunctional two dimensional coordination polymer. Adv. Mater. 2013, 25, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Rodriguez-Tapiador, M.I.; Castillo, O.; Olea, D.; Guijarro, A.; Alexandre, S.S.; Gomez-Herrero, J.; Zamora, F. Assembling of dimeric entities of Cd(II) with 6-mercaptopurine to afford one-dimensional coordination polymers: Synthesis and scanning probe microscopy characterization. Inorg. Chem. 2006, 45, 7642–7650. [Google Scholar] [CrossRef] [PubMed]
- Dubler, E.; Gyr, E. New metal-complexes of the antitumor drug 6-mercaptopurine—Syntheses and X-ray structural characterizations of dichloro(6-mercaptopurinium)copper(I), dichlorotetrakis(6-mercaptopurine)cadmium(II), and bis(6-mercaptopurinato)cadmium(II) dihydrate. Inorg. Chem. 1988, 27, 1466–1473. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. Wsxm: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Dong, X.Y.; Zhang, Z.J.; Pei, X.F.; Chen, X.J.; Chen, B.A.; Jin, J.A. Layered assembly of graphene oxide and co-al layered double hydroxide nanosheets as electrode materials for supercapacitors. Chem. Commun. 2011, 47, 3556–3558. [Google Scholar] [CrossRef] [PubMed]
- Kagunya, W.; Baddour-Hadjean, R.; Kooli, F.; Jones, W. Vibrational modes in layered double hydroxides and their calcined derivatives. Chem. Phys. 1998, 236, 225–234. [Google Scholar] [CrossRef]
- Williams, G.R.; O’Hare, D. Factors influencing staging during anion-exchange intercalation into [LiAl2(OH)6]X·mH2O (X = Cl−, Br−, NO3−). Chem. Mater. 2005, 17, 2632–2640. [Google Scholar] [CrossRef]
- Ijdo, W.L.; Pinnavaia, T.J. Staging of organic and inorganic gallery cations in layered silicate heterostructures. J. Solid State Chem. 1998, 139, 281–289. [Google Scholar] [CrossRef]
- Huang, G.L.; Ma, S.L.; Zhao, X.H.; Yang, X.J.; Ooi, K. Intercalation of bulk guest into ldh via osmotic swelling/restoration reaction: Control of the arrangements of thiacalix[4]arene anion intercalates. Chem. Mater. 2010, 22, 1870–1877. [Google Scholar] [CrossRef]
- Ma, S.L.; Wang, J.; Du, L.; Fan, C.H.; Sun, Y.H.; Sun, G.B.; Yang, X.J. Co-assembly of LDH nanosheets with crown ethers: Structural transformation and water-adsorption behavior. Eur. J. Inorg. Chem. 2013, 2013, 1363–1370. [Google Scholar] [CrossRef]
- Wypych, F.; Bubniak, G.A.; Halma, M.; Nakagaki, S. Exfoliation and immobilization of anionic iron porphyrin in layered double hydroxides. J. Colloid Interface Sci. 2003, 264, 203–207. [Google Scholar] [CrossRef]
- Nakagaki, S.; Halma, M.; Bail, A.; Arizaga, G.G.C.; Wypych, F. First insight into catalytic activity of anionic iron porphyrins immobilized on exfoliated layered double hydroxides. J. Colloid Interface Sci. 2005, 281, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L.; Goldstein, A.N.; Alivisatos, A.P. Semiconductor nanocrystals covalently bound to metal-surfaces with self-assembled monolayers. J. Am. Chem. Soc. 1992, 114, 5221–5230. [Google Scholar] [CrossRef]
- Profeti, L.P.R.; Dias, J.A.C.; Assaf, J.M.; Assaf, E.M. Hydrogen production by steam reforming of ethanol over Ni-based catalysts promoted with noble metals. J. Power Sources 2009, 190, 525–533. [Google Scholar] [CrossRef]
- Coronado, E.; Marti-Gastaldo, C.; Navarro-Moratalla, E.; Ribera, A.; Tatay, S. Illustrating the processability of magnetic layered double hydroxides: Layer-by-layer assembly of magnetic ultrathin films. Inorg. Chem. 2013, 52, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galan-Mascaros, J.R.; Marti-Gastaldo, C.; Ribera, A.; Palacios, E.; Castro, M.; Burriel, R. Spontaneous magnetization in Ni-Al and Ni-Fe layered double hydroxides. Inorg. Chem. 2008, 47, 9103–9110. [Google Scholar] [CrossRef] [PubMed]
- Abellan, G.; Busolo, F.; Coronado, E.; Marti-Gastaldo, C.; Ribera, A. Hybrid magnetic multilayers by intercalation of Cu(II) phthalocyanine in LDH hosts. J. Phys. Chem. C 2012, 116, 15756–15764. [Google Scholar] [CrossRef]
- Abellan, G.; Carrasco, J.A.; Coronado, E. Room temperature magnetism in layered double hydroxides due to magnetic nanoparticles. Inorg. Chem. 2013, 52, 7828–7830. [Google Scholar] [CrossRef] [PubMed]
- Abellan, G.; Coronado, E.; Marti-Gastaldo, C.; Ribera, A.; Jorda, J.L.; Garcia, H. Photo-switching in a hybrid material made of magnetic layered double hydroxides intercalated with azobenzene molecules. Adv. Mater. 2014, 26, 4156–4162. [Google Scholar] [CrossRef] [PubMed]
- Abellan, G.; Jorda, J.L.; Atienzar, P.; Varela, M.; Jaafar, M.; Gomez-Herrero, J.; Zamora, F.; Ribera, A.; Garcia, H.; Coronado, E. Stimuli-responsive hybrid materials: Breathing in magnetic layered double hydroxides induced by a thermoresponsive molecule. Chem. Sci. 2015, 6, 1949–1958. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abellán, G.; Amo-Ochoa, P.; Fierro, J.L.G.; Ribera, A.; Coronado, E.; Zamora, F. Self-Assembly of 1D/2D Hybrid Nanostructures Consisting of a Cd(II) Coordination Polymer and NiAl-Layered Double Hydroxides. Polymers 2016, 8, 5. https://doi.org/10.3390/polym8010005
Abellán G, Amo-Ochoa P, Fierro JLG, Ribera A, Coronado E, Zamora F. Self-Assembly of 1D/2D Hybrid Nanostructures Consisting of a Cd(II) Coordination Polymer and NiAl-Layered Double Hydroxides. Polymers. 2016; 8(1):5. https://doi.org/10.3390/polym8010005
Chicago/Turabian StyleAbellán, Gonzalo, Pilar Amo-Ochoa, José Luis G. Fierro, Antonio Ribera, Eugenio Coronado, and Félix Zamora. 2016. "Self-Assembly of 1D/2D Hybrid Nanostructures Consisting of a Cd(II) Coordination Polymer and NiAl-Layered Double Hydroxides" Polymers 8, no. 1: 5. https://doi.org/10.3390/polym8010005
APA StyleAbellán, G., Amo-Ochoa, P., Fierro, J. L. G., Ribera, A., Coronado, E., & Zamora, F. (2016). Self-Assembly of 1D/2D Hybrid Nanostructures Consisting of a Cd(II) Coordination Polymer and NiAl-Layered Double Hydroxides. Polymers, 8(1), 5. https://doi.org/10.3390/polym8010005