Microcapsules Filled with a Palm Oil-Based Alkyd as Healing Agent for Epoxy Matrix
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
2.2. Synthesis and Characterization of Palm Oil-Based Alkyd
2.3. Synthesis of Poly(melamine-urea-formaldehyde) (PMUF) Microcapsules with Alkyd Core
2.4. Characterization of Microcapsules
2.5. Morphology of Microcapsules
2.6. Thermal Analysis of Microcapsules
2.7. Microcapsules Dispersion in Epoxy Matrix
2.8. Flexural and Microhardness Tests
2.9. Reactions of Alkyd and Epoxy Matrix
3. Results and Discussion
3.1. Synthesis and Characterizations of Alkyd as Core Material
3.2. Synthesis of Microcapsules
3.3. Effect of Melamine Resin to Urea (M/U) Ratio
3.4. Spectroscopic Characterizations of Alkyd and Microcapsules
3.5. Thermal Analysis
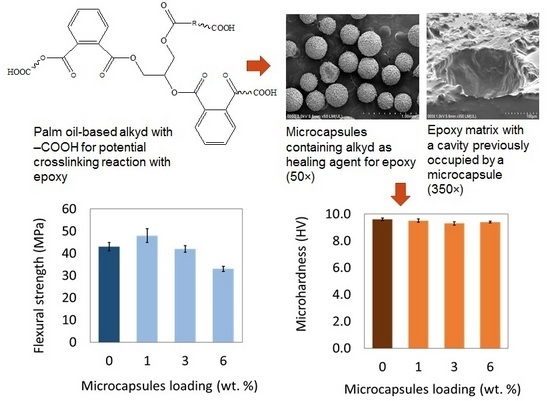
3.6. Morphology of Microcapsules and Epoxy Matrix
3.7. Flexural and Microhardness of Epoxy Matrix Loaded with 1%–6% Microcapsules
3.8. Investigating the Reactions of the Alkyd Blended with Epoxy Resin and Hardener at Different Ratios
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Caruso, M.M.; Delafuente, D.A.; Ho, V.; Sottos, N.R.; Moore, J.S.; White, S.R. Solvent-promoted self-healing epoxy materials. Macromolecules 2007, 40, 8830–8832. [Google Scholar] [CrossRef]
- Wilson, G.O.; Caruso, M.M.; Schelkopf, S.R.; Sottos, N.R.; White, S.R.; Moore, J.S. Adhesion promotion via noncovalent interactions in self-healing polymers. ACS Appl. Mater. Interfaces 2011, 3, 3072–3077. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, M.; Hine, P.J.; Khosravi, E. An autonomous self-healing system based on ROMP of norbornene dicarboximide monomers. Polymer 2012, 53, 5251–5257. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Boura, S.H.; Peikari, M.; Ashrafi, A.; Samadzadeh, M. Self-healing ability and adhesion strength of capsule embedded coatings—Micro and nano sized capsules containing linseed oil. Prog. Org. Coat. 2012, 75, 292–300. [Google Scholar] [CrossRef]
- Thanawala, K.; Mutneja, N.; Khanna, A.S.; Raman, R. Development of self-healing coatings based on linseed oil as autonomous repairing agent for corrosion resistance. Materials 2014, 7, 7324–7338. [Google Scholar] [CrossRef]
- Singha, A.; Thakur, V.K. Fabrication and characterization of H. sabdariffa fiber-reinforced green polymer composites. Polym. Plast. Technol. Eng. 2009, 48, 482–487. [Google Scholar] [CrossRef]
- Murphy, E.B.; Wudl, F. The world of smart healable materials. Prog. Polym. Sci. 2010, 35, 223–251. [Google Scholar] [CrossRef]
- Nesterova, T.; Dam-Johansen, K.; Kiil, S. Synthesis of durable microcapsules for self-healing anticorrosive coatings: A comparison of selected methods. Prog. Org. Coat. 2011, 70, 342–352. [Google Scholar] [CrossRef]
- Ullah, H.; Azizi, K.; Man, Z.B.; Ismail, M.B.C.; Khan, I. The potential of microencapsulated self-healing materials for microcracks recovery in self-healing composite systems: A review. Polym. Rev. 2016. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sheng, X.; Lee, J.K.; Kessler, M.R. Synthesis and characterization of melamine-urea-formaldehyde microcapsules containing ENB-based self-healing agents. Macromol. Mater. Eng. 2009, 294, 389–395. [Google Scholar] [CrossRef]
- Tong, X.M.; Zhang, T.; Yang, M.Z.; Zhang, Q. Preparation and characterization of novel melamine modified poly(urea-formaldehyde) self-repairing microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Then, S.; Gan, S.N.; Kasim, N.H.A. Performance of melamine modified urea-formaldehyde microcapsules in a dental host material. J. Appl. Polym. Sci. 2011, 122, 2557–2562. [Google Scholar] [CrossRef]
- Kienle, R.; Ferguson, C. Alkyd resins as film-forming materials. Ind. Eng. Chem. 1929, 21, 349–352. [Google Scholar] [CrossRef]
- Nabuurs, T.; Baijards, R.; German, A. Alkyd-acrylic hybrid systems for use as binders in waterborne paints. Prog. Org. Coat. 1996, 27, 163–172. [Google Scholar] [CrossRef]
- Azimi, A.; Yahya, R.; Gan, S.-N. Investigating effect of conventional and nano zinc pigments on air-drying property of palm-stearin-based alkyd resin paints. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 199–202. [Google Scholar] [CrossRef]
- Gan, S.N. Modification of Rubber by Alkyds. Malaysia Patent MY-143085-A, 15 March 2011. [Google Scholar]
- Lee, S.Y.; Gan, S.N.; Hassan, A.; Terakawa, K.; Hattori, T.; Ichikawa, N.; Choong, D.H. Reactions between epoxidized natural rubber and palm oil-based alkyds at ambient temperature. J. Appl. Polym. Sci. 2011, 120, 1503–1509. [Google Scholar] [CrossRef]
- Shahabudin, N.; Yahya, R.; Gan, S.N. Microencapsulation of a palm oil-based alkyd by amino resins. Macromol. Symp. 2015, 354, 305–313. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.; Xie, J.; Li, L.; Guo, J. Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Polymer 2006, 47, 5338–5349. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.X.; Wang, X.C.; Niu, J.J. Preparation and characterization of microencapsulated phase change material with low remnant formaldehyde content. Mater. Chem. Phys. 2007, 106, 437–442. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Facile fabrication of polystyrene/halloysite nanotube microspheres with core–shell structure via Pickering suspension polymerization. Polym. Bull. 2012, 69, 765–777. [Google Scholar] [CrossRef]
- Wang, X.; Xing, F.; Zhang, M.; Han, N.; Qian, Z. Experimental study on cementitious composites embedded with organic microcapsules. Materials 2013, 6, 4064–4081. [Google Scholar] [CrossRef]
- Kinloch, A.; Shaw, S.; Tod, D.; Hunston, D. Deformation and fracture behaviour of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Kinloch, A.; Mohammed, R.; Taylor, A.; Eger, C.; Sprenger, S.; Egan, D. The effect of silica nano particles and rubber particles on the toughness of multiphase thermosetting epoxy polymers. J. Mater. Sci. 2005, 40, 5083–5086. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q.; Chen, J.; Yang, G.C.; Li, X.M. Self-healing polymeric materials using epoxy/mercaptan as the healant. Macromolecules 2008, 41, 5197–5202. [Google Scholar] [CrossRef]


















| Details | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Ratio of alkyd:ENR50 | 1:9 | 1:1 | 9:1 |
| Initial observation | Clear solution | Clear solution | Clear solution |
| After 4 h | Turned viscous | Observable gel | Phase separation |
| Upon removal of toluene | Sticky mass | Elastic solid | Brittle solid |
| Characteristic | AlkPKO65 |
|---|---|
| Oil length (%) | 65 |
| Acid number (mg KOH·g−1) | 15 |
| Viscosity (Pa·s) | 2.14 |
| Sample | M/U Ratio | M (g) | U (g) | Yield (%) | Core-Content (wt %) | Mean Diameter (µm) | Description of Microcapsules (MCs) |
|---|---|---|---|---|---|---|---|
| A2 | 0 | 0 | 2.50 | 40 | 89.9 (0.5) | 403 (56) | Spherical, free-flowing |
| B1 | 0.03 | 0.08 | 2.49 | 65 | 94.8 (0.3) | 383 (56) | Spherical, free-flowing |
| B2 | 0.06 | 0.16 | 2.47 | 60 | 92.0 (1.3) | 380 (60) | Spherical, free-flowing |
| B3 | 0.12 | 0.30 | 2.45 | 49 | 91.9 (0.4) | 384 (55) | Spherical, free-flowing |
| B4 | 0.29 | 0.70 | 2.40 | – | – | – | Mixture of irregular shapes |
| Sample | Td Onset (°C) | T50% (°C) |
|---|---|---|
| A2 | 250 | 352 |
| B1 | 250 | 375 |
| B2 | 258 | 375 |
| B3 | 245 | 369 |
| Alkyd | 250 | 342 |
| PUF shell | 220 | 310 |
| PMUF shell | 220 | 331 |
| Sample | Eq. wt Ratio of Epoxy/Amine/Alkyd | Epoxy/Alkyd wt Ratio | Epoxy (g) | Amine (g) | Alkyd (g) | After 24 h at rt |
|---|---|---|---|---|---|---|
| Control | 1/1/0 | 100/0 | 1 | 0.58 | 0 | Cured, solid |
| EA1 | 1/0.8/0.2 | 100/39 | 1 | 0.44 | 0.39 | Cured, solid |
| EA2 | 1/0.8/0.1 | 100/20 | 1 | 0.44 | 0.20 | Cured, solid |
| EA3 | 1/0.7/0.1 | 100/20 | 1 | 0.39 | 0.20 | Cured, solid |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahabudin, N.; Yahya, R.; Gan, S.N. Microcapsules Filled with a Palm Oil-Based Alkyd as Healing Agent for Epoxy Matrix. Polymers 2016, 8, 125. https://doi.org/10.3390/polym8040125
Shahabudin N, Yahya R, Gan SN. Microcapsules Filled with a Palm Oil-Based Alkyd as Healing Agent for Epoxy Matrix. Polymers. 2016; 8(4):125. https://doi.org/10.3390/polym8040125
Chicago/Turabian StyleShahabudin, Nurshafiza, Rosiyah Yahya, and Seng Neon Gan. 2016. "Microcapsules Filled with a Palm Oil-Based Alkyd as Healing Agent for Epoxy Matrix" Polymers 8, no. 4: 125. https://doi.org/10.3390/polym8040125
APA StyleShahabudin, N., Yahya, R., & Gan, S. N. (2016). Microcapsules Filled with a Palm Oil-Based Alkyd as Healing Agent for Epoxy Matrix. Polymers, 8(4), 125. https://doi.org/10.3390/polym8040125