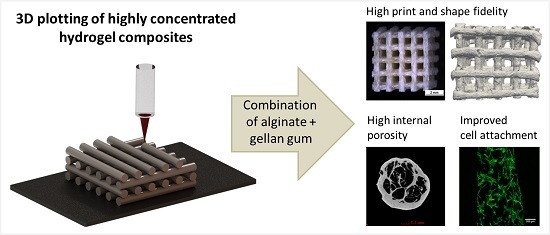
Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Plotting Pastes and Viscosity Measurements
2.2. Scaffold Fabrication
2.3. Scaffold Characterization
2.4. Cultivation of hMSC on the Plotted Scaffolds
2.4.1. Cell Seeding and Osteogenic Differentiation
2.4.2. Biochemical Analysis of Cell Proliferation and Osteogenic Differentiation
2.4.3. Confocal Laser Scanning Microscopy (cLSM)
2.5. Statistics
3. Results
3.1. Properties of the Composite Pastes
3.2. Characterization of the Scaffolds
3.3. Cell Culture
4. Discussion
5. Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klimaszewska, K. Plantlet development from immature zygotic embryos of hybrid larch through somatic embryogenesis. Plant Sci. 1989, 63, 95–103. [Google Scholar] [CrossRef]
- Rule, P.L.; Alexander, A.D. Gellan gum substitute for agar in leptospiral media. J. Clin. Microbiol. 1986, 23, 500–504. [Google Scholar]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.J.; Gilmore, K.J.; Wallace, G.G.; Panhuis, M. In het modified gellan gum hydrogels for tissue engineering applications. Soft Matter 2013, 9, 3705–3711. [Google Scholar] [CrossRef]
- Tako, M.; Teruya, T.; Tamaki, Y.; Konishi, T. Molecular origin for rheological characteristics of native gellan gum. Colloid Polym. Sci. 2009, 287, 1445–1454. [Google Scholar] [CrossRef]
- Lee, H.; Fisher, S.; Kallos, M.S.; Hunter, C.J. Optimizing gelling parameters of gellan gum for fibrocartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98B, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Santos, T.C.; Martins, L.; Picciochi, R.; Marques, A.P.; Castro, A.G.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum injectable hydrogels for cartilage tissue engineering applications: In vitro studies and preliminary in vivo evaluation. Tissue Eng. Part A 2010, 16, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, N.A.; Salgado, A.J.; Sousa, R.A.; Oliveira, J.T.; Pedro, A.J.; Leite-Almeida, H.; Cerqueira, R.; Almeida, A.; Mastronardi, F.; Mano, J.F. Development and characterization of a novel hybrid tissue engineering-based scaffold for spinal cord injury repair. Tissue Eng. Part A 2009, 16, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Landers, R.; Mülhaupt, R. Desktop manufacturing of complex objects, prototypes and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromol. Mater. Eng. 2000, 282, 17–21. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J. A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan Gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Lode, A.; Sonntag, F.; Nies, B.; Gelinsky, M. Well-ordered biphasic calcium phosphate–alginate scaffolds fabricated by multi-channel 3D plotting under mild conditions. J. Mater. Chem. B 2013, 1, 4088–4098. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Gelinsky, M. Direct plotting of three-dimensional hollow fiber scaffolds based on concentrated alginate pastes for tissue engineering. Adv. Healthc. Mater. 2013, 2, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Wehrl, M.; Paul, B.; Hochmuth, T.; Schumacher, M.; Schütz, K.; Gelinsky, M. Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS ONE 2015, 10, e0129205. [Google Scholar] [CrossRef] [PubMed]
- Despang, F.; Bernhardt, A.; Lode, A.; Dittrich, R.; Hanke, T.; Shenoy, S.J.; Mani, S.; John, A.; Gelinsky, M. Synthesis and physicochemical, in vitro and in vivo evaluation of an anisotropic, nanocrystalline hydroxyapatite bisque scaffold with parallel-aligned pores mimicking the microstructure of cortical bone. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Schütz, K.; Placht, A.-M.; Paul, B.; Brüggemeier, S.; Gelinsky, M.; Lode, A. Three-dimensional plotting of a cell-laden alginate/methylcellulose blend: Towards biofabrication of tissue engineering constructs with clinically relevant dimensions. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High mechanical strength double-network hydrogel with bacterial cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effects of surface functional groups on protein adsorption and subsequent cell adhesion using self-assembled monolayers. J. Mater. Chem. 2007, 17, 4079. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.J.; In het Panhuis, M. Conducting bio-materials based on gellan gum hydrogels. Soft Matter 2009, 5, 3430–3437. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ohno, J.; Sato, A.; Kido, H.; Fukushima, T. Mesenchymal stem cell spheroids exhibit enhanced in vitro and in vivo osteoregenerative potential. BMC Biotechnol. 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, A.; Planell, J.A.; Engel, E. Extracellular calcium and CaSR drive osteoinduction in mesenchymal stromal cells. Acta Biomater. 2014, 10, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Rojo, L.; Gentleman, E.; Sardinha, J.P.; Stevens, M.M. Strontium- and zinc-alginate hydrogels for bone tissue engineering. Tissue Eng. Part A 2011, 17, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements—Approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef]
- Gantar, A.; da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Krawczyk, G.; Pamula, E.; Declercq, H.A.; Schaubroeck, D.; Bucko, M.M.; Balcaen, L.; van der Voort, P.; Bliznuk, V.; van den Vreken, N.M.F.; et al. Generation of composites for bone tissue-engineering applications consisting of gellan gum hydrogels mineralized with calcium and magnesium phosphate phases by enzymatic means. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Pilarz, M.; Lopez-Heredia, M.; Brackman, G.; Schaubroeck, D.; Balcaen, L.; Bliznuk, V.; Dubruel, P.; Knabe-Ducheyne, C.; Vanhaecke, F.; et al. Composites of gellan gum hydrogel enzymatically mineralized with calcium-zinc phosphate for bone regeneration with antibacterial activity. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]








| Material | Average viscosity (Pa s) (at constant shear rate: 10 s−1) | Extrusion pressure (kPa) |
|---|---|---|
| PA | 530.2 ± 6.3 | 480 |
| AGG2 | 738.6 ± 10.4 | 520 |
| AGG3 | 843.2 ± 9.9 | 530 |
| Scaffold type | Porosity measured by pycnometer (n = 5) (%) | Porosity measured by µCT image analysis (n = 4) (%) |
|---|---|---|
| PA | 79.7 ± 1.2 | 66.9 ± 3.4 |
| AGG2 | 69.5 ± 1.2 ** | 60.8 ± 2.8 |
| AGG3 | 69.3 ± 1.0 ** | 62.3 ± 2.3 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkineni, A.R.; Ahlfeld, T.; Funk, A.; Waske, A.; Lode, A.; Gelinsky, M. Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds. Polymers 2016, 8, 170. https://doi.org/10.3390/polym8050170
Akkineni AR, Ahlfeld T, Funk A, Waske A, Lode A, Gelinsky M. Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds. Polymers. 2016; 8(5):170. https://doi.org/10.3390/polym8050170
Chicago/Turabian StyleAkkineni, Ashwini Rahul, Tilman Ahlfeld, Alexander Funk, Anja Waske, Anja Lode, and Michael Gelinsky. 2016. "Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds" Polymers 8, no. 5: 170. https://doi.org/10.3390/polym8050170
APA StyleAkkineni, A. R., Ahlfeld, T., Funk, A., Waske, A., Lode, A., & Gelinsky, M. (2016). Highly Concentrated Alginate-Gellan Gum Composites for 3D Plotting of Complex Tissue Engineering Scaffolds. Polymers, 8(5), 170. https://doi.org/10.3390/polym8050170