Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells
Abstract
:1. Introduction
2. Experimental Methods
2.1. Reagents and Apparatus
2.2. Degradation of Polysaccharides
2.3. Average Molecular Weight Determination
2.4. Measurement of Protein Content
2.4.1. Ultraviolet-Absorption Method
2.4.2. Ninhydrin Method
2.5. Analysis of Sulfate-Group Content
2.6. Analysis of Carboxylic Group Content
2.7. Fourier Transform Infrared Spectrometry
2.8. Cytotoxicity Measurement of Polysaccharides
2.9. Repair Effect of Polysaccharide on Damaged HK-2 Cells
2.10. Hematoxyline-Eosin Staining
2.11. Statistical Analysis
3. Results and Discussion
3.1. Degradation of Polysaccharides
3.2. Chemical Characteristics
3.3. FT-IR Spectroscopy
3.4. Cytotoxicity of Different SPSs on HK-2 Cells
3.5. Repair Ability of Different SPSs on Damaged HK-2 Cells
3.5.1. Effect of Polysaccharide Concentration
3.5.2. Effect of –SO3H Content
3.5.3. Effect of Molecular Weight
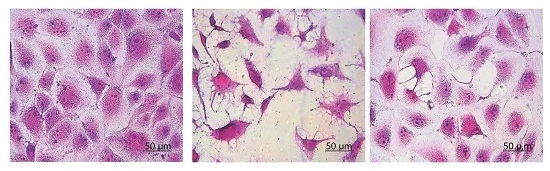
3.6. Morphological Changes of HK-2 Cells after Repair by Different Polysaccharides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, S.; Gao, X.; Sun, Y.; Xu, C.; Wang, L.; Zhou, T. Analysis of HK-2 cells exposed to oxalate and calcium oxalate crystals: Proteomic insights into the molecular mechanisms of renal injury and stone formation. Urol. Res. 2010, 38, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.M.; Boppana, N.B.; Asokan, D.; Sekaran, S.D.; Shankar, E.M.; Li, C.; Gopal, K.; Bakar, S.A.; Karthik, H.S.; Ebrahim, A.S. C-phycocyanin confers protection against oxalate-mediated oxidative stress and mitochondrial dysfunctions in MDCK cells. PLoS ONE 2014, 9, 93056–93064. [Google Scholar] [CrossRef] [PubMed]
- Boeve, E.R.; Cao, L.C.; Verkoelen, C.F.; Romijn, J.C.; de Bruijin, W.C.; Schroder, F.H. Glycosaminoglycans and other sulphated polysaccharides in calculogenesis of urinary stones. World J. Urol. 1994, 12, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.-M.; Wang, M.; Lu, P.; Tan, J. Degradation of sulfated polysaccharide extracted from algal Laminaria japonica and its modulation on calcium oxalate crystallization. Mater. Sci. Eng. C 2010, 30, 1022–1029. [Google Scholar] [CrossRef]
- Veena, C.K.; Josephine, A.; Preetha, S.P.; Varalakshmi, P.; Sundarapandiyan, R. Renal peroxidative changes mediated by oxalate: The protective role of fucoidan. Life Sci. 2006, 79, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Kong, T.; Wu, W.-H.; Lan, M.-B. The protection of polysaccharide from the brown seaweed Sargassum graminifolium against ethylene glycol-induced mitochondrial damage. Mar. Drugs 2013, 11, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Morabito, M.; Armeli, M.S.; Lo, P.G.; Pagano, M.; Genovese, G. Potential use of polysaccharides from the brown alga Undaria pinnatifida as anticoagulants. Braz. Arch. Biol. Technol. 2015, 58, 798–804. [Google Scholar] [CrossRef]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.-T.; Zhang, Q.-B.; Qi, H.-M.; Zhang, H.; Niu, X.-Z.; Xu, Z.-H.; Li, Z.-E. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xue, C.-H.; Li, B.-F. Study of antioxidant activities of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2008, 20, 431–436. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Zhang, Q.B.; Wang, J.; Song, H.F.; Zhang, H.; Niu, X.Z. Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from Porphyra haitanensis. Carbohydr. Polym. 2010, 79, 290–295. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xie, J.-H.; Kan, L.-J.; Wang, J.-Q.; Shen, M.-Y.; Li, W.-J.; Nie, S.-P.; Xie, M.-Y. Sulfated polysaccharides from Cyclocary apaliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2015, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.-Q.; Nie, S.-P.; Wang, Y.-X.; Cui, S.W.; Xie, M.-Y. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015, 79, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, H.; Zhang, J.; Wang, X.; Zhao, B.; Yao, J.; Wang, Y. Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydr. Polym. 2010, 8, 897–905. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Watcharatanyatip, K.; Homvises, T.; Jaturongkakul, K.; Thongboonkerd, V. Systematic comparisons of various spectrophotometric and colorimetric methods to measure concentrations of protein, peptide and amino acid: Detectable limits, linear dynamic ranges, interferences, practicality and unit costs. Talanta 2012, 98, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Pandima Devi, K. Evaluation of physicochemical properties, proximate and nutritional composition of Gracilaria edulis collected from palk bay. Food Chem. 2015, 174, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, M.-Y.; Xu, L.; Lian, W.; Xiang, J.-Y.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.-M.; Zhao, J.-H. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spanò, A.; Morabito, M.; Maugeri, T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the Straits of Messina against pathogens relevant in aquacultureq. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Faggio, C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Fu, H.; Wang, M.; Deng, C.; Song, Z.; Chen, J. Immunomodulatory activity of heparan sulfate mimetics from Escherichia coli K5 capsular polysaccharide in vitro. Carbohydr. Polym. 2015, 115, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Ouyang, J.-M.; Zhu, W.-Y.; Li, Y.-B.; Gan, Q.-Z. Size-Dependent toxicity and interactions of calcium oxalate dihydrate crystal on Vero renal epithelial cells. J. Mater. Chem. B 2015, 3, 1864–1878. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Mo, X.; Qi, H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum(Doll.) Ching. Carbohydr. Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Li, J.; Liu, H. Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem. 2014, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akahane, T.; Takeuchi, S.; Minakata, A. Conductimetric titration of polyelectrolytes having sulfate and carboxyl groups. Polym. Bull. 1990, 24, 437–444. [Google Scholar] [CrossRef]
- Ge, Q.; Mao, J.-W.; Zhang, A.-Q.; Wang, Y.-J.; Sun, P.-L. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilát). Food Sci. Biotechnol. 2013, 22, 301–307. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightiion WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Chemical characterization and ameliorating effect of polysaccharide from Chinese jujube on intestine oxidative injury by ischemia and reperfusion. Int. J. Biol. Macromol. 2011, 48, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.-F.; Ji, Y.-B.; Meng, D.-Y. Sulfated modifiation and anti‑tumor activity of laminarin. Experimen. Therapeut. Med. 2013, 6, 1259–1264. [Google Scholar]
- Schuchmann, M.N.; von Sonntag, C. Effect of oxygen on OH-radical-induced scission of glycosidic linkage of cellobiose. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1978, 34, 397–400. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Dore, C.M.P.G.; Alves, M.G.C.F.; Santos, N.D.; Cruz, A.K.M.; Câmara, R.B.G.; Castro, A.J.G.; Alves, L.G.; Nader, H.B.; Leite, E.L. Antiangiogenic activity and direct antitumor effect from a sulfated polysaccharide isolated from seaweed. Microvasc. Res. 2013, 88, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, Y.; Li, S.; Huang, L.; Chen, W.; Lin, X. Promotion proliferation effect of a polysaccharide from Aloe barbadensis Miller on human fibroblasts in vitro. Int. J. Biol. Macromol. 2009, 45, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Schepers, M.S.J.; Van Ballegooijen, E.S.; Bangma, C.H.; Verkoelen, C.F. Oxalate is toxic to renal tubular cells only at supraphysiologic concentrations. Kidney Int. 2005, 68, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-Q.; Ouyang, J.-M.; Peng, H.; Zhu, W.-Y.; Chen, H.-Q. Inhibition on calcium oxalate crystallization and repair on injured renal epithelial cells of degraded soybean polysaccharide. Carbohydr. Polym. 2012, 90, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, Q.; Zhang, Z.; Wang, J. In vitro antioxidant activity of polysaccharides extracted from Bryopsis plumosa. Carbohydr. Polym. 2010, 80, 1057–1061. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]







| Polysaccharide species | Concentration of H2O2 | Constant of Mark-Houwink equation | Intrinsic viscosity η (mL/g) | ||
|---|---|---|---|---|---|
| κ | α | Before degradation | After degradation | ||
| LJ-1 | – | 8.21 × 10−3 | 0.782 | 3.1 | – |
| PY-2 | 12% | 7.86 × 10−3 | 0.626 | 5.1 | 1.4 |
| GL-3 | 10% | 7.00 × 10−2 | 0.720 | 1,039 | 24.1 |
| SF-4 | 12% | 1.92 × 10−4 | 1.23 | 26.3 | 4.9 |
| EU-5 | – | 1.92 × 10−4 | 1.23 | 3.4 | – |
| UP-6 | 15% | 8.21 × 10−3 | 0.782 | 1460 | 4.9 |
| SPSs | Mean molecular weights M/Da | –SO3H contents/% | –COOH contents/% | Uronic acid contents/% | ||||
|---|---|---|---|---|---|---|---|---|
| BD * | AD * | BD | AD | BD | AD | BD | AD | |
| Laminaria-1 | 1,968 | – | 21.7 | – | 1.2 | – | 5.2 | – |
| Porphyra-2 | 3.1 × 104 | 4,020 | 17.3 | 17.9 | 0.9 | 1.7 | 3.9 | 7.3 |
| Gracilaria-3 | 6.2 × 105 | 3,343 | 12.7 | 13.3 | 1.0 | 1.0 | 4.3 | 4.3 |
| Sargassum-4 | 1.6 × 104 | 3,828 | 8.4 | 8.2 | 1.4 | 1.3 | 6.0 | 5.4 |
| Eucheuma-5 | 2,850 | – | 7.0 | – | 1.2 | – | 5.0 | – |
| Undaria-6 | 5.2 × 106 | 3,635 | 6.1 | 5.5 | 1.3 | 1.2 | 5.6 | 5.2 |
| SPSs | –SO3H contents/% | –COOH contents/% | Characteristic absorption peaks of groups/cm−1 | |||
|---|---|---|---|---|---|---|
| –OH | –COOH | –OSO3 | Sugar ring | |||
| LJ-1 | 21.7 | 1.2 | 3,400.9 | 1,632.1 | 1,262.3 | 2,912.2, 1,460, 1,380.3, 1,082.5, 1,019.4, 881.2 |
| PY-2 | 17.3 | 0.9 | 3,436.3 | 1,632.9 | 1,249.2 | 2,922.0, 1,383.3, 1,080.9 |
| DPY-2 | 17.9 | 1.7 | 3,429.2 | 1,618.4 | 1,250.0 | 2,925.5, 1,384.9, 1,080.8 |
| GL-3 | 12.7 | 1.0 | 3,419.2 | 1,641.1 | 1,257.2 | 2,921.5, 1,378.4, 1,024.3 |
| DGL-3 | 13.3 | 1.0 | 3,415.3 | 1,614.7 | 1,261.1 | 2,921.4, 1,383.8, 1,025.6 |
| SF-4 | 8.4 | 1.4 | 3,415.1 | 1,630.9 | 1,260.2 | 2,931.8, 1,460.0, 1,380.2, 1,084.4, 1,021.6, 928.1 |
| DSF-4 | 8.2 | 1.3 | 3,423.3 | 1,637.3 | 1,247.7 | 2,927.0, 1,421.2, 1,382.5, 1,081.2, 1,024.0, 935.3 |
| EU-5 | 7.0 | 1.2 | 3,399.9 | 1,629.5 | 1,256.3 | 2,939.6, 1,459.1, 1,381.1, 1,086.9, 1,022.0, 931.8 |
| UP-6 | 6.1 | 1.3 | 3,419.4 | 1,632.7 | 1,262.3 | 2,917.3, 1,022.5 |
| DUP-6 | 5.5 | 1.2 | 3,413.2 | 1,615.8 | 1,262.8 | 2,921.4, 1,024.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhadja, P.; Tan, C.-Y.; Ouyang, J.-M.; Yu, K. Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers 2016, 8, 188. https://doi.org/10.3390/polym8050188
Bhadja P, Tan C-Y, Ouyang J-M, Yu K. Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers. 2016; 8(5):188. https://doi.org/10.3390/polym8050188
Chicago/Turabian StyleBhadja, Poonam, Cai-Yan Tan, Jian-Ming Ouyang, and Kai Yu. 2016. "Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells" Polymers 8, no. 5: 188. https://doi.org/10.3390/polym8050188
APA StyleBhadja, P., Tan, C. -Y., Ouyang, J. -M., & Yu, K. (2016). Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers, 8(5), 188. https://doi.org/10.3390/polym8050188