RAFT-Mediated Polymerization-Induced Self-Assembly of Poly(Acrylic Acid)-b-Poly(Hexafluorobutyl Acrylate): Effect of the pH on the Synthesis of Self-Stabilized Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Poly(Acrylic Acid) Macro-RAFT Agent
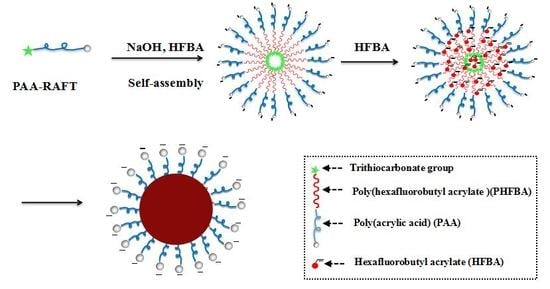
2.2. RAFT Emulsion Polymerization of Hexafluorobutyl Acrylate Mediated by Poly(Acrylic Acid) Macro-RAFT Agent
2.3. 1H-Nuclear Magnetic Resonance (1H-NMR)
2.4. Size Exclusion Chromatography (SEC)
2.5. Dynamic Light Scattering (DLS)
2.6. Transmission Electron Microscopy (TEM)
2.7. pH Monitoring
3. Results
3.1. Synthesis of Poly(Acrylic Acid) Macro-RAFT Agent by Solution Polymerization in Ethanol
3.2. RAFT Emulsion Polymerization of Hexafluorobutyl Acrylate Using PAA Macro-RAFT Agent
3.3. Morphology and Size of PAA-b-PHFBA Latex Particles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, G.; Zheng, H.; Wang, Y.; Wang, H.; Dong, Q.; Bai, R. A facile strategy for the fabrication of highly stable superhydrophobic cotton fabric using amphiphilic fluorinated triblock azide copolymers. Polymer 2010, 51, 1940–1946. [Google Scholar] [CrossRef]
- Zhen, W.; He, L.; Liang, J.; Chang, G.; Wang, N. Preparation and properties of core-shell nanosilica/poly(methyl methacrylate-butyl acrylate-2,2,2-trifluoroethyl methacrylate) latex. J. Appl. Polym. Sci. 2011, 120, 1152–1161. [Google Scholar]
- Cui, X.; Zhong, S.; Yan, J.; Wang, C.; Zhang, H.; Wang, H. Synthesis and characterization of core-shell SiO2-fluorinated polyacrylate nanocomposite latex particles containing fluorine in the shell. Colloids Surf. A 2010, 360, 41–46. [Google Scholar] [CrossRef]
- Qu, A.; Wen, X.; Pi, P.; Cheng, J.; Yang, Z. Synthesis and characterization of hybrid fluoro-emulsion based on silica/copolymer composite particles. Polym. Int. 2008, 57, 1287–1294. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.R.; Li, H.Q. Preparation and characterization of soap-free fluorine-containing acrylate latex. J. Coat. Technol. Res. 2010, 7, 469–476. [Google Scholar] [CrossRef]
- Bruno, A. Controlled radical (co) polymerization of fluoromonomers. Macromolecules 2010, 43, 10163–10184. [Google Scholar] [CrossRef]
- Sauer, R.; Froimowicz, P.; Schöller, K.; Cramer, J.M.; Ritz, S.; Mailänder, V.; Landfester, K. Design, synthesis and miniemulsion polymerization of new phosphonate surfmers and application studies of the resulting nanoparticles as model systems for biomimetic mineralization and cellular uptake. Chem. Eur. J. 2012, 18, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Eastoe, J.; Paul, A.; Downer, A.; Steytler, D.C.; Rumsey, E. Effects of fluorocarbon surfactant chain structure on stability of water-in-carbon dioxide microemulsions. Links between aqueous surface tension and microemulsion stability. Langmuir 2002, 18, 3014–3017. [Google Scholar] [CrossRef]
- Dreher, W.R.; Jarrett, W.L.; Urban, M.W. Stable nonspherical fluorine-containing colloidal dispersions: Synthesis and film formation. Macromolecules 2005, 38, 2205–2212. [Google Scholar] [CrossRef]
- Linemann, R.F.; Malner, T.E.; Brandsch, R.; Bar, G.; Ritter, W.; Mülhaupt, R. Latex blends of fluorinated and fluorine-free acrylates: Emulsion polymerization and tapping mode atomic force microscopy of film formation. Macromolecules 1999, 32, 1715–1721. [Google Scholar] [CrossRef]
- Misra, A.; Urban, M.W. Environmentally compliant fluoro-containing MMA/nBA colloidal dispersions; synthesis, molecular modeling, and coalescence. Macromolecules 2009, 42, 7828–7835. [Google Scholar] [CrossRef]
- Delaittre, G.; Save, M.; Charleux, B. Nitroxide-mediated aqueous dispersion polymerization: From water-soluble macroalkoxyamine to thermosensitive nanogels. Macromol. Rapid Commun. 2007, 28, 1528–1533. [Google Scholar] [CrossRef]
- Brusseau, S.; Belleney, J.; Magnet, S.; Couvreur, L.; Charleux, B. Nitroxide-mediated copolymerization of methacrylic acid with sodium 4-styrene sulfonate: Towards new water-soluble macroalkoxyamines for the synthesis of amphiphilic block copolymers and nanoparticles. Polym. Chem. 2010, 1, 720–729. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, Y.; Fu, Z.; Yang, W. Synthesis of amphiphilic poly(methyl methacrylate)-block-poly(methacrylic acid) diblock copolymers by atom transfer radical polymerization. Polym. Int. 2006, 55, 360–364. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Li, B.G.; Zhu, S. Toward well-controlled ab initio RAFT emulsion polymerization of styrene mediated by 2-(((dodecylsulfanyl)carbonothioyl)sulfanyl) propanoic acid. Macromolecules 2010, 44, 221–229. [Google Scholar] [CrossRef]
- Yeole, N.; Hundiwale, D.; Jana, T. Synthesis of core-shell polystyrene nanoparticles by surfactant free emulsion polymerization using macro-RAFT agent. J. Colloid Interface Sci. 2011, 354, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.R.; Rodríguez-Hernández, A.A.; Hernández, N.; Castillo, P.; Saldívar, E.; Ríos, L. Controlled/living free radical copolymerization of styrene and butyl acrylate in bulk and emulsion with industrial monomers. Influence of monomer addition on polymer properties. Ind. Eng. Chem. Res. 2005, 44, 2792–2801. [Google Scholar] [CrossRef]
- Zhang, W.; D’Agosto, F.; Boyron, O.; Rieger, J.; Charleux, B. One-pot synthesis of poly(methacrylic acid-co-poly(ethylene oxide)methyl ether methacrylate)-b-polystyrene amphiphilic block copolymers and their self-assemblies in water via RAFT-mediated radical emulsion polymerization. A kinetic study. Macromolecules 2011, 44, 7584–7593. [Google Scholar] [CrossRef]
- Sprong, E.; Leswin, J.S.; Lamb, D.J.; Ferguson, C.J.; Hawkett, B.S.; Pham, B.T.; Gilbert, R.G. Molecular watchmaking: Ab initio emulsion polymerization by RAFT-controlled self-assembly. Macromol. Symp. 2005, 231, 84–93. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.; Ryan, A.J.; Armes, S.P. From a water-immiscible monomer to block copolymer nano-objects via a one-pot RAFT aqueous dispersion polymerization formulation. Macromolecules 2013, 46, 769–777. [Google Scholar] [CrossRef]
- Manguian, M.; Save, M.; Charleux, B. Batch emulsion polymerization of styrene stabilized by a hydrophilic macro-RAFT agent. Macromol. Rapid Commun. 2006, 27, 399–404. [Google Scholar] [CrossRef]
- Wi, Y.; Lee, K.; Lee, B.H.; Choe, S. Soap-free emulsion polymerization of styrene using poly(methacrylic acid) macro-RAFT agent. Polymer 2008, 49, 5626–5635. [Google Scholar] [CrossRef]
- Chaduc, I.; Lansalot, M.; D’Agosto, F.; Charleux, B. RAFT polymerization of methacrylic acid in water. Macromolecules 2012, 45, 1241–1247. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Pham, B.T.; Hawkett, B.S.; Gilbert, R.G.; Serelis, A.K.; Such, C.H. Effective ab initio emulsion polymerization under RAFT control. Macromolecules 2002, 35, 9243–9245. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Hughes, R.J.; Nguyen, D.; Pham, B.T.; Gilbert, R.G.; Serelis, A.K.; Hawkett, B.S. Ab initio emulsion polymerization by RAFT-controlled self-assembly §. Macromolecules 2005, 38, 2191–2204. [Google Scholar] [CrossRef]
- Bar-Nes, G.; Hall, R.; Sharma, V.; Gaborieau, M.; Lucas, D.; Castignolles, P.; Gilbert, R.G. Controlled/living radical polymerization of isoprene and butadiene in emulsion. Eur. Polym. J. 2009, 45, 3149–3163. [Google Scholar] [CrossRef]
- Ganeva, D.E.; Sprong, E.; de Bruyn, H.; Warr, G.G.; Such, C.H.; Hawkett, B.S. Particle formation in ab initio RAFT mediated emulsion polymerization systems. Macromolecules 2007, 40, 6181–6189. [Google Scholar] [CrossRef]
- Chenal, M.; Bouteiller, L.; Rieger, J. Ab initio RAFT emulsion polymerization of butyl acrylate mediated by poly(acrylic acid) trithiocarbonate. Polym. Chem. 2013, 4, 752–762. [Google Scholar] [CrossRef]
- Chaduc, I.; Girod, M.; Antoine, R.; Charleux, B.; D’Agosto, F.; Lansalot, M. Batch emulsion polymerization mediated by poly(methacrylic acid) macroRAFT agents: One-pot synthesis of self-stabilized particles. Macromolecules 2012, 45, 5881–5893. [Google Scholar] [CrossRef]
- Guo, L.; Jiang, Y.; Qiu, T.; Meng, Y.; Li, X. One-pot synthesis of poly(methacrylic acid)-b-poly(2,2,2-trifluoroethyl methacrylate) diblock copolymers via RAFT polymerization. Polymer 2014, 55, 4601–4610. [Google Scholar] [CrossRef]
- Such, C.H.; Rizzardo, E.; Serelis, A.K.; Hawkett, B.S.; Gilbert, R.G.; Ferguson, C.J.; Hughes, R.J. Aqueous dispersions of polymer particles. U.S. Patent 20060223936A1, 2006. [Google Scholar]
- Jiang, X.; Lu, G.; Feng, C.; Li, Y.; Huang, X. Poly(acrylic acid)-graft-poly(N-vinylcaprolactam): A novel pH and thermo dual-stimuli responsive system. Polym. Chem. 2013, 4, 3876–3884. [Google Scholar] [CrossRef]
- Sun, J.T.; Hong, C.Y.; Pan, C.Y. Formation of the block copolymer aggregates via polymerization-induced self-assembly and reorganization. Soft Matter 2012, 8, 7753–7767. [Google Scholar] [CrossRef]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic insights for block copolymer morphologies: How do worms form vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Blanazs, A.; Armes, S.P.; Ryan, A.J.; Lewis, A.L. Aqueous dispersion polymerization: A new paradigm for in situ block copolymer self-assembly in concentrated solution. J. Am. Chem. Soc. 2011, 133, 15707–15713. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Ryan, A.J.; Armes, S.P. Predictive phase diagrams for RAFT aqueous dispersion polymerization: Effect of block copolymer composition, molecular weight, and copolymer concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]
- Blanazs, A.; Verber, R.; Mykhaylyk, O.O.; Ryan, A.J.; Heath, J.Z.; Douglas, C.I.; Armes, S.P. Sterilizable gels from thermoresponsive block copolymer worms. J. Am. Chem. Soc. 2012, 134, 9741–9748. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P.; Blanazs, A.; Battaglia, G.; Armes, S.P. Facile synthesis of methacrylic ABC triblock copolymer vesicles by RAFT aqueous dispersion polymerization. Macromolecules 2012, 45, 5081–5090. [Google Scholar] [CrossRef]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-induced self-assembly: From soluble macromolecules to block copolymer nano-objects in one step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Bozovic-Vukic, J.; Mañon, H.T.; Meuldijk, J.; Koning, C.; Klumperman, B. SAN-b-P4VP block copolymer synthesis by chain extension from RAFT-functional poly(4-vinylpyridine) in solution and in emulsion. Macromolecules 2007, 40, 7132–7139. [Google Scholar] [CrossRef]
- Warren, N.J.; Armes, S.P. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185. [Google Scholar] [CrossRef] [PubMed]
- Derry, M.J.; Fielding, L.A.; Armes, S.P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. [Google Scholar] [CrossRef]
- Warren, N.J.; Mykhaylyk, O.O.; Ryan, A.J.; Williams, M.; Doussineau, T.; Dugourd, P.; Armes, S.P. Testing the vesicular morphology to destruction: Birth and death of diblock copolymer vesicles prepared via polymerization-induced self-assembly. J. Am. Chem. Soc. 2015, 137, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Bai, Y.; Zhang, X.; Zhang, L. Room temperature synthesis of poly(poly(ethylene glycol)methyl ether methacrylate)-based diblock copolymer nano-objects via photoinitiated polymerization-induced self-assembly (photo-PISA). Polym. Chem. 2016, 7, 2372–2380. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.; McKenzie, B.E.; Le Bouëdec, G.M.; Williams, C.N.; Brown, S.L.; Armes, S.P. Polymerization-induced self-assembly of all-acrylic diblock copolymers via RAFT dispersion polymerization in alkanes. Macromolecules 2015, 48, 8594–8607. [Google Scholar] [CrossRef]
- Chaduc, I.; Zhang, W.; Rieger, J.; Lansalot, M.; D’Agosto, F.; Charleux, B. Amphiphilic block copolymers from a direct and one-pot RAFT synthesis in water. Macromol. Rapid Commun. 2011, 32, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Chen, Y.; Zhang, C. Synthesis of fluorinated gradient copolymers by RAFT emulsifier-free emulsion polymerization and their compatibilization in copolymer blends. Colloid Polym. Sci. 2014, 292, 2803–2809. [Google Scholar] [CrossRef]
- Zhang, W.; D’Agosto, F.; Boyron, O.; Rieger, J.; Charleux, B. Toward a better understanding of the parameters that lead to the formation of nonspherical polystyrene particles via RAFT-mediated one-pot aqueous emulsion polymerization. Macromolecules 2012, 45, 4075–4084. [Google Scholar] [CrossRef]
- Zhang, W.; D’Agosto, F.; Dugas, P.Y.; Rieger, J.; Charleux, B. RAFT-mediated one-pot aqueous emulsion polymerization of methyl methacrylate in presence of poly(methacrylic acid-co-poly(ethylene oxide)methacrylate) trithiocarbonate macromolecular chain transfer agent. Polymer 2013, 54, 2011–2019. [Google Scholar] [CrossRef]
- Cho, C.; Wallace, K.L.; Hagen, D.A.; Stevens, B.; Regev, O.; Grunlan, J.C. Nanobrick wall multilayer thin films grown faster and stronger using electrophoretic deposition. Nanotechnology 2015, 26, 185703. [Google Scholar] [CrossRef] [PubMed]
- Convertine, A.J.; Lokitz, B.S.; Lowe, A.B.; Scales, C.W.; Myrick, L.J.; McCormick, C.L. Aqueous RAFT polymerization of acrylamide and N,N-dimethylacrylamide at room temperature. Macromol. Rapid Commun. 2005, 26, 791–795. [Google Scholar] [CrossRef]
- Liang, J.; He, L.; Zheng, Y. Synthesis and property investigation of three core-shell fluoroacrylate copolymer latexes. J. Appl. Polym. Sci. 2009, 112, 1615–1621. [Google Scholar]
- Cui, X.; Zhong, S.; Wang, H. Emulsifier-free core-shell polyacrylate latex nanoparticles containing fluorine and silicon in shell. Polymer 2007, 48, 7241–7248. [Google Scholar] [CrossRef]
- Neugebauer, D.; Sumerlin, B.S.; Matyjaszewski, K.; Goodhart, B.; Sheiko, S.S. How dense are cylindrical brushes grafted from a multifunctional macroinitiator? Polymer 2004, 45, 8173–8179. [Google Scholar] [CrossRef]
- Zang, L.; Guo, J.; Luo, J.; Zhang, H. Synthesis and characterization of fluorine-containing polyacrylate latex with core-shell structure by UV-initiated seeded emulsion polymerization. Polym. Adv. Technol. 2012, 23, 15–20. [Google Scholar] [CrossRef]









| Sample a | pH | Conversion b (%) | Mn c (g·mol−1) | ĐM c |
|---|---|---|---|---|
| Latex 1 | 2.58 | 53.12 | 1.02 × 104 | 1.017 |
| Latex 2 | 4.55 | 56.24 | 1.13 × 104 | 1.023 |
| Latex 3 | 6.82 | 65.12 | 1.20 × 104 | 1.021 |
| Latex 4 | 7.36 | 85.61 | 1.23 × 104 | 1.021 |
| Latex 5 | 8.13 | 89.92 | 1.24 × 104 | 1.021 |
| Latex 6 | 9.36 | 90.23 | 1.26 × 104 | 1.024 |
| Latex 7 | 10.55 | 92.32 | 1.30 × 104 | 1.423 |
| Latex 8 | 11.55 | 94.12 | 3.93 × 105 | 1.817 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; He, R.; Ma, J. RAFT-Mediated Polymerization-Induced Self-Assembly of Poly(Acrylic Acid)-b-Poly(Hexafluorobutyl Acrylate): Effect of the pH on the Synthesis of Self-Stabilized Particles. Polymers 2016, 8, 207. https://doi.org/10.3390/polym8060207
Zhou J, He R, Ma J. RAFT-Mediated Polymerization-Induced Self-Assembly of Poly(Acrylic Acid)-b-Poly(Hexafluorobutyl Acrylate): Effect of the pH on the Synthesis of Self-Stabilized Particles. Polymers. 2016; 8(6):207. https://doi.org/10.3390/polym8060207
Chicago/Turabian StyleZhou, Jianhua, Renyan He, and Jianzhong Ma. 2016. "RAFT-Mediated Polymerization-Induced Self-Assembly of Poly(Acrylic Acid)-b-Poly(Hexafluorobutyl Acrylate): Effect of the pH on the Synthesis of Self-Stabilized Particles" Polymers 8, no. 6: 207. https://doi.org/10.3390/polym8060207
APA StyleZhou, J., He, R., & Ma, J. (2016). RAFT-Mediated Polymerization-Induced Self-Assembly of Poly(Acrylic Acid)-b-Poly(Hexafluorobutyl Acrylate): Effect of the pH on the Synthesis of Self-Stabilized Particles. Polymers, 8(6), 207. https://doi.org/10.3390/polym8060207