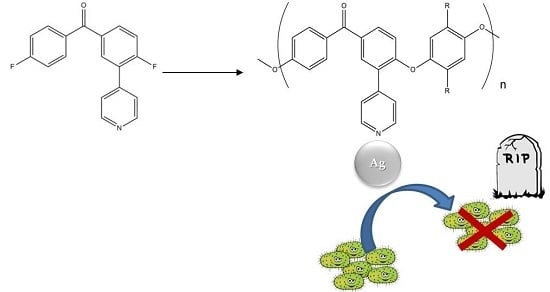
Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 3-Bromo-4-Fluorobenzoic Acid (2)
2.3. Preparation of 3-Bromo-4,4′-Difluorobenzophenone (3)
2.4. Preparation of 3-Pyridin-4-yl-4,4′-Difluorobenzophenone (4)
2.5. Preparation of 4-Pyridin-4-yl-4,4′-Difluorobenzophenone (5)
2.6. Preparation of 3-Pyridin-4-yl-4,4′-Difluorobenzophenone Silver Trifluoromethanesulfonate Complex (4a)
2.7. Preparation of 3-Pyridin-4-yl-4,4′-Difluorobenzophenone Silver Methanoate Complex (4b)
2.8. Preparation of 3-Pyridin-3-yl-4-4′-Difluorobenzophenone Silver Nitrate Complex (5a)
2.9. General Procedure for Polymerization of Polyether-Ether-Ketone-3-Pyridiny-4-yl (PEEKNx = PEEKN5, PEEKN6 and PEEKN7)
2.10. Coating of PEEKNx with Ethanediyl Bis(isoniconate) Silver Nitrate Complex
2.11. Antimicrobial Assay
3. Results and Discussion
3.1. X-Ray Diffraction
3.2. Polymerisation
3.3. Coating of PEEKN with Silver Species
3.4. Antimicrobial Properties of PEEK Derivatives
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PEEK | Polyether Ether Ketone |
| THF | Tetrahydrofuran |
| EtOH | Ethanol |
| NMR | Nuclear Magnetic Resonance |
| IR | Infra-red |
| MS | Mass Spectroscopy |
| TGA | Thermal Gravimetric Analysis |
| DSC | Differential Scanning Calorimetry |
| XRPD | X-ray Powder Diffractogram |
| DMTA | Dynamic Mechanical Thermal Analysis |
| ICP | Induced Coupled Plasma |
| SEM | Scanning Electron Microscopy |
| Tg | Glass Transition Temperature |
| Tm | Melting Temperature |
References
- Wei, J.; Lu, R.; Liu, F. Novel, highly efficient polymeric benzophenone photoinitiator containing coinitiator moieties for photopolymerization. Polym. Adv. Technol. 2010, 21, 656–662. [Google Scholar] [CrossRef]
- Papilloud, S.; Baudraz, D. Analysis of food packaging UV inks for chemicals with potential to migrate into food simulants. Food Addit. Contam. 2002, 19, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Staniland, P.A. Thermoplastische aromatische polyetherketone. EP0184458 A2, 11 June 1986. [Google Scholar]
- Van der Vegt, A.; Govaert, L. Plymeren van Keten tot Kunststof, 5th ed.; DUP Blue Print: Delft, The Netherlands, 2003. [Google Scholar]
- Seferis, J.C. Polyetheretherketone (PEEK): Processing-structure and properties studies for a matrix in high performance composites. Polym. Compos. 1986, 7, 158–169. [Google Scholar] [CrossRef]
- Corvelli, A.A.; Roberts, J.C.; Biermann, P.J.; Cranmer, J.H. Characterization of a peek composite segmental bone replacement implant. J. Mater. Sci. 1999, 34, 2421–2431. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef] [Green Version]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.J.; Sagers, R.D.; Pitt, W.G. Bacterial adhesion to orthopedic implant polymers. J. Biomed. Mater. Res. 1996, 30, 403–410. [Google Scholar] [CrossRef]
- Vaudaux, P.; François, P.; Berger-Bächi, B.; Lew, D.P. In vivo emergence of subpopulations expressing teicoplanin or vancomycin resistance phenotypes in a glycopeptide-susceptible, methicillin-resistant strain of Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 47, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Vig Slenters, T.; Sague, J.L.; Brunetto, P.S.; Zuber, S.; Fleury, A.; Mirolo, L.; Robin, A.Y.; Meuwly, M.; Gordon, O.; Landmann, R.; et al. Of chains and rings: Synthetic strategies and theoretical investigations for tuning the structure of silver coordination compounds and their applications. Materials 2010, 3, 3407–3429. [Google Scholar] [CrossRef]
- Fromm, K.M. Give silver a shine. Nat. Chem. 2011, 3, 178. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, P.S.; Vig Slenters, T.; Fromm, K.M. In vitro biocompatibility of new silver(I) coordination compound coated-surfaces for dental implant applications. Materials 2011, 4, 355–367. [Google Scholar] [CrossRef]
- Fromm, K.M. Silver coordination compounds with antimicrobial properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Varisco, M.; Khanna, N.; Brunetto, P.S.; Fromm, K.M. New antimicrobial and biocompatible implant coating with synergic Silver-Vancomycin conjugate action. ChemMedChem 2014, 9, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Brunetto, P.S.; Braissant, O.; Rajacic, Z.; Khanna, N.; Landmann, R.; Daniels, A.U.; Fromm, K.M. Development of a polystyrene sulfonate/silver nanocomposite with self-healing properties for biomaterial applications. Comptes Rendus Chim. 2013, 16, 550–556. [Google Scholar] [CrossRef]
- Kamm, O.; Mattheww, A.O. p-Nitrobenzoic acid. Org. Synth. 1922, 2, 53. [Google Scholar]
- Weikert, R.J.; Bingham, S.; Emanuel, M.A.; Fraser Smith, E.B.; Loughhead, D.G.; Nelson, P.H.; Poulton, A.L. Synthesis and anthelmintic activity of 3′-benzoylurea derivatives of 6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]t hiazole+. J. Med. Chem. 1991, 34, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Gors, H.G.; Horner, P.J.; Jansons, V. Friedel-crafts preparation of aromatic ketones. U.S. Patent 4814508 A, 21 March 1989. [Google Scholar]
- Cosier, J.; Glazer, A.M. A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J. Appl. Cryst. 1986, 19, 105–107. [Google Scholar] [CrossRef]
- Blanc, E.; Schwarzenbach, D.; Flack, H.D. The evaluation of transmission factors and their first derivatives with respect to crystal shape parameters. J. Appl. Cryst. 1991, 24, 1035–1041. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; de Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-97. Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Hoffmann, R.; Swenson, J.R. Ground- and excited-state geometries of benzophenone. J. Phys. Chem. 1970, 74, 415–420. [Google Scholar] [CrossRef]
- Maginn, S.J.; Davey, R.J. 4,4′-difluorobenzophenone. Acta Crystallogr. Sect. C 1994, 50, 254–255. [Google Scholar] [CrossRef]
- Wu, L.-L.; Yang, C.-L.; Lo, F.-C.; Chiang, C.-H.; Chang, C.-W.; Ng, K.Y.; Chou, H.-H.; Hung, H.-Y.; Chan, S.I.; Yu, S.S.-F. Tuning the regio- and stereoselectivity of C–H activation in n-octanes by cytochrome P450 BM-3 with fluorine substituents: Evidence for interactions between a C–F bond and aromatic π systems. Chem. Eur. J. 2011, 17, 4774–4787. [Google Scholar] [CrossRef] [PubMed]
- Laia, Y.H.; Kuoa, M.C.; Huanga, J.C.; Chena, M. On the PEEK composites reinforced by surface-modified nano-silica. Mater. Sci. Eng. A 2007, 458, 158–169. [Google Scholar] [CrossRef]
- Johansson, M.; Malmström, E.; Hult, A. Synthesis, characterization, and curing of hyperbranched allyl ether-maleate functional ester resins. J. Polym. Sci. A 1993, 31, 619–624. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Gómez-Fatou, M.A. Mechanical and electrical properties of novel poly(ether ether ketone)/carbon nanotube/inorganic fullerene-like WS2 hybrid nanocomposites: experimental measurements and theoretical predictions. Mater. Chem. Phys. 2011, 130, 126–133. [Google Scholar] [CrossRef]
- Paulik, F.; Paulik, J.; Arnold, M. Examination of the decomposition of AgNO3 by means of simultaneous EGA and TG method under conventional and quasi isothermal circumstances. Thermochim. Acta 1985, 92, 787–790. [Google Scholar] [CrossRef]
















| PEEKN Derivative | Glass Transition Temperature °C | Melting Temperature °C | Decomposition Temperature °C |
|---|---|---|---|
| PEEK [4] | 140 | 340 | >400 |
| PEEKN5 | 190 | 230 | 380 |
| PEEKN7 | 165 | 270 | 380 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, J.; Joset, N.; Crochet, A.; Tan, M.; Holzheu, A.; Brunetto, P.S.; Fromm, K.M. Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts. Polymers 2016, 8, 208. https://doi.org/10.3390/polym8060208
Girard J, Joset N, Crochet A, Tan M, Holzheu A, Brunetto PS, Fromm KM. Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts. Polymers. 2016; 8(6):208. https://doi.org/10.3390/polym8060208
Chicago/Turabian StyleGirard, Jérôme, Nathalie Joset, Aurélien Crochet, Milène Tan, Anja Holzheu, Priscilla S. Brunetto, and Katharina M. Fromm. 2016. "Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts" Polymers 8, no. 6: 208. https://doi.org/10.3390/polym8060208
APA StyleGirard, J., Joset, N., Crochet, A., Tan, M., Holzheu, A., Brunetto, P. S., & Fromm, K. M. (2016). Synthesis of New Polyether Ether Ketone Derivatives with Silver Binding Site and Coordination Compounds of Their Monomers with Different Silver Salts. Polymers, 8(6), 208. https://doi.org/10.3390/polym8060208