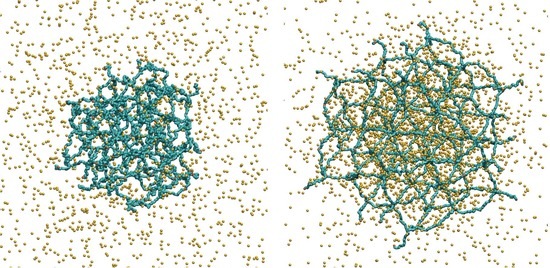
Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results
Abstract
:1. Introduction
2. Model
3. Theoretical Estimation of Gel Size
4. Simulation Results: Comparison with Analytical Theory
4.1. Salt-Free Microgel
4.2. Microgel in Presence of Salt
5. Simulation Results: Radial Microgel Properties
5.1. Radial Monomer Distribution
5.2. Radial Polymer Conformations
5.3. Radial Counterion Distribution
5.4. Radial Effective Charge
5.5. Monomer-Counterion Pair Distribution Function
6. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Ilmain, F.; Tanaka, T.; Kokufuta, E. Volume transition in a gel driven by hydrogen bonding. Nature 1991, 349, 400–401. [Google Scholar] [CrossRef]
- Das, M.; Zhang, H.; Kumacheva, E. Microgels: Old materials with new applications. Annu. Rev. Mater. Res. 2006, 36, 117–142. [Google Scholar] [CrossRef]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147–148, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Tam, K.C. Review on the dynamics and micro-structure of pH-responsive nano-colloidal systems. Adv. Colloid Interface Sci. 2008, 136, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid Polym. Sci. 2011, 289, 625–646. [Google Scholar] [CrossRef]
- Scherzinger, C.; Holderer, O.; Richter, D.; Richtering, W. Polymer dynamics in responsive microgels: Influence of cononsolvency and microgel architecture. Phys. Chem. Chem. Phys. 2012, 14, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Button, B.; Cai, L.H.; Ehre, C.; Kesimer, M.; Hill, D.B.; Sheehan, J.K.; Boucher, R.C.; Rubinstein, M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012, 337, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Linse, P. Monte Carlo simulation of defect-free cross-linked polyelectrolyte gels. J. Phys. Chem. B 2003, 32, 8030–8040. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Hentschke, R. Computer simulation study on the swelling of a polyelectrolyte gel by a Stockmayer solvent. Phys. Rev. E 2003, 67, 061807. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; de Pablo, J.J. Monte Carlo simulation of a coarse-grained model of polyelectrolyte networks. Phys. Rev. Lett. 2003, 91, 018301. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.A.; Everaers, R.; Holm, C.; Kremer, K. Scaling in polyelectrolyte networks. Europhys. Lett. 2004, 67, 786–792. [Google Scholar] [CrossRef]
- Edgecombe, S.; Linse, P. Monte Carlo simulations of cross-linked polyelectrolyte gels with oppositely charged macroions. Langmuir 2006, 22, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.A.; Holm, C.; Kremer, K. Swelling of polyelectrolyte networks. J. Chem. Phys. 2005, 122, 154903. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.W.; Horkay, F.; Douglas, J.F.; de Pablo, J.J. Molecular simulation of the swelling of polyelectrolyte gels by monovalent and divalent counterions. J. Chem. Phys. 2008, 129, 154902. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Peres, M.; Maroto-Centeno, J.A.; Martin-Molina, A. Effect of the counterion valence on the behavior of thermo-sensitive gels and microgels: A Monte Carlo simulation study. Macromolecules 2012, 45, 8872–8879. [Google Scholar] [CrossRef]
- Košovan, P.; Richter, T.; Holm, C. Molecular simulations of hydrogels. In Intelligent Hydrogels; Springer: Basel, Switzerland, 2013; pp. 205–221. [Google Scholar]
- Claudio, G.C.; Kremer, K.; Holm, C.J. Comparison of a hydrogel model to the Poisson-Boltzmann cell model. J. Chem. Phys. 2009, 131, 094903. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Zwanikken, J.W.; Detcheverry, F.A.; de Pablo, J.J.; de la Cruz, M.O. Study of volume phase transitions in polymeric nanogels by theoretically informed coarse-grained simulations. Soft Matter 2011, 7, 5965–5975. [Google Scholar] [CrossRef]
- Jha, P.K.; Zwanikken, J.W.; de Pablo, J.J.; de la Cruz, M.O. Electrostatic control of nanoscale phase behavior of polyelectrolyte networks. Curr. Opin. Solid State Mater. Sci. 2011, 15, 271–276. [Google Scholar] [CrossRef]
- Kramarenko, E.Y.; Khokhlov, A.R.; Yoshikawa, K. Collapse of polyelectrolyte macromolecules revisited. Macromolecules 1997, 30, 3383–3388. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Ahualli, S.; Martín-Molina, A. Temperature-sensitive nanogels in the presence of salt: Explicit coarse-grained simulations. J. Chem. Phys. 2014, 141, 124903. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Winkler, R.G. Universal conformational properties of polymers in ionic nanogels. Sci. Rep. 2016, 6, 19836. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.R. Counterion penetration and effective electrostatic interactions in solutions of polyelectrolyte stars and microgels. Phys. Rev. E 2003, 67, 011804. [Google Scholar] [CrossRef] [PubMed]
- Denton, A.R.; Tang, Q. Counterion-induced swelling of ionic microgels. J. Chem. Phys. 2016, 145, 164901. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Guenoun, P.; Muller, F.; Belloni, L.; Delsanti, M. Monte Carlo simulations of star-branched polyelectrolyte micelles. Eur. Phys. J. E 2002, 9, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.G.; Gold, M.; Reineker, P. Collapse of polyelectrolyte macromolecules by counterion condensation and ion pair formation: A molecular dynamics simulation study. Phys. Rev. Lett. 1998, 80, 3731–3734. [Google Scholar] [CrossRef]
- Gompper, G.; Ihle, T.; Kroll, D.M.; Winkler, R.G. Multi-Particle Collision Dynamics: A particle-based mesoscale simulation approach to the hydrodynamics of complex Fluids. Adv. Polym. Sci. 2009, 221, 1–87. [Google Scholar]
- Ripoll, M.; Winkler, R.G.; Gompper, G. Hydrodynamic screening of star polymers in shear flow. Eur. Phys. J. E 2007, 23, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mussawisade, K.; Ripoll, M.; Winkler, R.G.; Gompper, G. Dynamics of polymers in a particle based mesoscopic solvent. J. Chem. Phys. 2005, 123, 144905. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Winkler, R.G. Structure of microgels with Debye–Hückel interactions. Polymers 2014, 6, 1602–1617. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, R.; Rudov, A.A.; Lyon, L.A.; Richtering, W.; Pich, A.; Potemkin, I.I. Electrostatic interactions and osmotic pressure of counterions control the pH-dependent swelling and collapse of polyampholyte microgels with random distribution of ionizable groups. Macromolecules 2015, 48, 5914–5927. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Pippig, M.; Potts, D. Particle simulation based on nonequispaced fast Fourier transforms. In Fast Methods for Long-Range Interactions in Complex Systems, 6th ed.; Sutmann, G., Gibbon, P., Lippert, T., Eds.; IAS-Series; Forschungszentrum Jülich: Jülich, Germany, 2011; pp. 131–158. [Google Scholar]
- Pippig, M.; Potts, D. Parallel three-dimensional nonequispaced fast Fourier transforms and their application to particle simulation. SIAM J. Sci. Comput. 2013, 35, C411–C437. [Google Scholar] [CrossRef]
- ScaFaCoS. Available online: https://github.com/scafacos (accessed on 10 August 2015).
- Arnold, A.; Fahrenberger, F.; Holm, C.; Lenz, O.; Bolten, M.; Dachsel, H.; Halver, R.; Kabadshow, I.; Gähler, F.; Heber, F.; et al. Comparison of scalable fast methods for long-range interactions. Phys. Rev. E 2013, 88, 063308. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.G. Flow Simulations with Multiparticle Collision Dynamics. In Hierarchical Methods for Dynamics in Complex Molecular Systems; Grotendorst, J., Sutmann, G., Gompper, G., Marx, D.E., Eds.; IAS Series; Forschungszentrum Jülich: Jülich, Germany, 2012; Volume 10. [Google Scholar]
- Kapral, R. Multiparticle Collision Dynamics: Simulations of complex systems on mesoscale. Adv. Chem. Phys. 2008, 140, 89–146. [Google Scholar]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- Barrat, J.L.; Joanny, J.F. Theory of polyelectrolyte solutions. Adv. Chem. Phys. 1997, 94, 66. [Google Scholar]
- Barrat, J.L.; Joanny, J.F.; Pincus, P. On the scattering properties of polyelectrolyte gels. J. Phys. II 1992, 2, 1531–1544. [Google Scholar] [CrossRef]
- Nisato, G.; Munch, J.; Candau, S. Swelling, structure, and elasticity of polyampholyte hydrogels. Langmuir 1999, 15, 4236–4244. [Google Scholar] [CrossRef]
- López-León, T.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Elaïssari, A. Cationic and anionic poly (N-isopropylacrylamide) based submicron gel particles: Electrokinetic properties and colloidal stability. J. Phys. Chem. B 2006, 110, 4629–4636. [Google Scholar] [CrossRef] [PubMed]
- Capriles-González, D.; Sierra-Martín, B.; Fernández-Nieves, A.; Fernández-Barbero, A. Coupled deswelling of multiresponse microgels. J. Phys. Chem. B 2008, 112, 12195–12200. [Google Scholar] [CrossRef] [PubMed]
- Colla, T.; Likos, C.N.; Levin, Y. Equilibrium properties of charged microgels: A Poisson-Boltzmann-Flory approach. J. Chem. Phys. 2014, 141, 234902. [Google Scholar] [CrossRef] [PubMed]
- Košovan, P.; Richter, T.; Holm, C. Modeling of Polyelectrolyte Gels in Equilibrium with Salt Solutions. Macromolecules 2015, 48, 7698–7708. [Google Scholar] [CrossRef]
- Jusufi, A.; Likos, C.; Löwen, H. Counterion-induced entropic interactions in solutions of strongly stretched, osmotic polyelectrolyte stars. J. Chem. Phys. 2002, 116, 11011–11027. [Google Scholar] [CrossRef]
- Hofmann, T.; Winkler, R.G.; Reineker, P. Integral equation theory approach to rodlike polyelectrolytes: Counterion condensation. J. Chem. Phys. 2001, 114, 10181. [Google Scholar] [CrossRef]















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Halver, R.; Sutmann, G.; Winkler, R.G. Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results. Polymers 2017, 9, 15. https://doi.org/10.3390/polym9010015
Kobayashi H, Halver R, Sutmann G, Winkler RG. Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results. Polymers. 2017; 9(1):15. https://doi.org/10.3390/polym9010015
Chicago/Turabian StyleKobayashi, Hideki, Rene Halver, Godehard Sutmann, and Roland G. Winkler. 2017. "Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results" Polymers 9, no. 1: 15. https://doi.org/10.3390/polym9010015
APA StyleKobayashi, H., Halver, R., Sutmann, G., & Winkler, R. G. (2017). Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results. Polymers, 9(1), 15. https://doi.org/10.3390/polym9010015