Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Monomer
2.2.1. Synthesis of 4-(4-Nitrophenoxy)acetophenone (NPAP)
2.2.2. Synthesis of 4-(4-(1-Pyrrolidinyl)phenyl)-2,6-bis(4-(4-nitrophenoxy)phenyl)pyridine (PPNPP)
2.2.3. Synthesis of 4-(4-(1-Pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine (PPAPP)
2.3. Preparation of Poly(Amic Acid) (PAA)
2.4. Preparation of PI Films
2.5. Characterization Methods
2.5.1. Fourier Transform Infrared (FT-IR)
2.5.2. Nuclear Magnetic Resonance (NMR)
2.5.3. Mass Spectrometry (MS)
2.5.4. Thermo-Gravimetric Analysis (TGA)
2.5.5. Differential Scanning Calorimetry (DSC)
2.5.6. Solubility Analysis
2.5.7. The Contact Angle
2.5.8. The Wide-Angle X-ray Diffraction (WAXD)
2.5.9. Inherent Viscosities
3. Results and Discussion
3.1. Monomer Synthesis
3.2. Polymer Synthesis
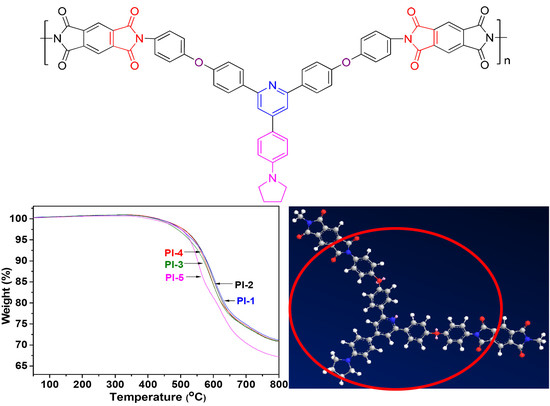
3.3. Thermal Properties
3.4. Solubility Properties
3.5. Hydrophobic Properties
3.6. WAXD Data
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, M.X. Isomeric polyimides. Prog. Polym. Sci. 2007, 32, 623–668. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ghosh, A.; Sen, S.K.; Banerjee, S.; Voit, B. ChemInform abstract: solubility improvements in aromatic polyimides by macromolecular engineering. RSC Adv. 2012, 2, 5900–5926. [Google Scholar] [CrossRef]
- Yi, L.; Huang, W.; Yan, D.Y. Soluble aromatic polyimides with high glass transition temperature from benzidine containing tert-butyl groups. J. Polym. Sci. A 2017, 55, 533–559. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Ghaemy, M.; Berenjestanaki, F.R.; Bazzar, M. Organosoluble, thermally stable and low dielectric constant fluorinated polyimides containing 2,4,5-triphenylimidazole moiety in the main chains. Des. Monomers Polym. 2013, 17, 101–110. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, C.; Wang, D.; Dang, G.; Chen, C.; Zhou, H.; Zhao, X. High transparent polyimides containing pyridine and biphenyl units: Synthesis, thermal, mechanical, crystal and optical properties. Polymer 2015, 62, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, M.; Yi, L.; Wang, Y. High glass transition of organo-soluble copolyimides derived from a rigid diamine with tert-butyl-substituted triphenylpyridine moiety. RSC Adv. 2013, 3, 7271–7276. [Google Scholar] [CrossRef]
- Roy, A.; Hickner, M.A.; Lee, H.S.; Glass, T.; Paul, M.; Badami, A.; Riffle, J.S.; McGrath, J.E. States of water in proton exchange membranes: Part A—Influence of chemical structure and composition. Polymer 2017, 111, 297–306. [Google Scholar] [CrossRef]
- Miki, M.; Horiuchi, H.; Yamada, Y. Synthesis and gas transport properties of hyperbranched polyimide-silica hybrid/composite membranes. Polymers 2013, 5, 1362–1379. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Fan, W.; Liu, J.; Wang, Z.; Li, G. Thermosetting polyimides and composites based on highly soluble phenylethynyl-terminated isoimide oligomers. RSC Adv. 2014, 4, 37458–37469. [Google Scholar] [CrossRef]
- Shojo, D.; Yamazaki, S.; Kimura, K. Hydrothermal synthesis of aromatic polyimide particles by using reaction-induced crystallization. J. Polym. Sci. A 2015, 53, 2795–2799. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Mechref, E.; Jabbour, J.; Calas-Etienne, S.; Amro, K.; Mehdi, A.; Tauk, R.; Zaouk, D.; Etienne, P. Synthesis and characterization of a photosensitive organic-inorganic, hybrid positive resin type material: Application to the manufacture of microfluidic devices by laser writing. RSC Adv. 2016, 6, 3951–3959. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, N.; Shen, K.; Song, N.; Shi, K.; Zhu, S.; Zhang, Y.; Guan, S. From a flexible hyperbranched polyimide to a microporous polyimide network: Microporous architecture and carbon dioxide adsorption. Polymer 2017, 115, 176–183. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, F.; Lai, J.; Qi, H.; You, X. A new series of fluorinated alicyclic-functionalized polyimides derivated from natural-(D)-camphor: Synthesis, structure–properties relationships and dynamic dielectric analyses. Polymer 2013, 54, 5673–5683. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Geydt, P.; Bugrov, A.N.; Ovaska, S.S.; Lahderanta, E.; Toikka, A.M. Structure and transport properties of mixed-matrix membranes based on polyimides with ZrO2 nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Zhao, H.; Song, L.; Fang, X. Synthesis and properties of transparent polyimides derived from trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride. RSC Adv. 2015, 5, 53926–53934. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; He, M.; Wang, H.; Cui, Y.; Zhang, T. Synthesis and characterization of fluorinated polyimides derived from 1,4-bis-[4-amino-2-(trifluoromethyl)-phenoxy] benzene/tetrafluoride benzene. Des. Monomers Polym. 2014, 17, 590–600. [Google Scholar] [CrossRef]
- Wen, P.; He, R.; Li, X.D.; Lee, M.H. Syntheses and characterizations of high refractive index and low birefringence polyimides containing spirobifluorene in the side chain. Polymer 2017, 117, 76–83. [Google Scholar] [CrossRef]
- Wang, F.; Shao, L.S.; Bai, Q.Y.; Che, X.; Liu, B.; Wang, Y.H. Photo-induced vertical alignment of liquid crystals via in situ polymerization initiated by polyimide containing benzophenone. Polymers 2017, 9, 233. [Google Scholar] [CrossRef]
- Xia, S.; Sun, Z.; Yi, L.; Wang, Y. Synthesis of soluble polyimide derived from novel naphthalene diamines for liquid crystal alignment layers and a preliminary study on the mechanism of imidization. RSC Adv. 2013, 3, 14661–14670. [Google Scholar] [CrossRef]
- Sun, N.W.; Meng, S.Y.; Zhou, Z.W.; Yao, J.N.; Du, Y.L.; Wang, D.M.; Zhao, X.G.; Zhou, H.W.; Chen, C.H. High-contrast electrochromic and electrofluorescent dual-switching materials based on 2-diphenylamine-(9,9-diphenylfluorene)-functionalized semi-aromatic polymers. RSC Adv. 2016, 6, 66288–66296. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, L.; Zhu, Y.L.; Song, Y.J.; Wang, L.J.; Shen, Y.Z. Synthesis and memory characteristics of novel soluble polyimides based on asymmetrical diamines containing carbazole. Polymer 2016, 91, 118–127. [Google Scholar] [CrossRef]
- Chen, B.K.; Wu, T.Y.; Wong, J.M.; Chang, Y.M.; Lee, H.F.; Huang, W.Y.; Chen, A. Highly sulfonated diamine synthesized polyimides and protic ionic liquid composite membranes improve PEM conductivity. Polymers 2015, 7, 1046–1065. [Google Scholar] [CrossRef]
- Van Genabet, B.; Schwarz, A.; Bruneel, E.; Rambausek, L.; Van Driessche, I.; Van Langenhove, L. Synthesis and characterization of copper, polyimide and TIPS-pentacene layers for the development of a solution processed fibrous transistor. AIP Adv. 2011, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.C.; Jung, J.W.; Choi, J.Y.; Chung, C.M. Kinetic study of low-temperature imidization of poly(amic acid)s and preparation of colorless, transparent polyimide films. J. Polym. Sci. A 2016, 54, 1593–1602. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Gong, C.; Zhang, S.; Ma, T. Synthesis and characterization of novel soluble pyridine-containing polyimides based on 4-phenyl-2,6-bis 4-(4-aminophenoxy)phenyl-pyridine and various aromatic dianhydrides. J. Appl. Polym. Sci. 2007, 104, 212–219. [Google Scholar] [CrossRef]
- Wozniak, A.I.; Yegorov, A.S.; Ivanov, V.S.; Igumnov, S.M.; Tcarkova, K.V. Recent progress in synthesis of fluorine containing monomers for polyimides. J. Fluor. Chem. 2015, 180, 45–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Zhang, Q.Y.; Xu, Z.S.; Yeung, K.W.K.; Yi, C.F. Review on F, Si and P-containing polyimides with special properties. Sci. Adv. Mater. 2014, 6, 44–55. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cao, S.J.; Chen, W.T.; Xu, C.; Zhao, X.Y.; Li, J.; Ren, Q. Synthesis and properties of fluorinated polyimides with multi-bulky pendant groups. RSC Adv. 2017, 7, 26420–26427. [Google Scholar] [CrossRef]
- Liu, C.J.; Pei, X.L.; Mei, M.; Chou, G.Q.; Huang, X.H.; Wei, C. Synthesis and characterization of organosoluble, transparent, and hydrophobic fluorinated polyimides derived from 3,3-diisopropyl-4,4-diaminodiphenyl-4-trifluoromethyltoluene. High Perform. Polym. 2016, 28, 1114–1123. [Google Scholar] [CrossRef]
- Lei, R.; Kang, C.Q.; Huang, Y.J.; Qiu, X.P.; Ji, X.I.; Xing, W.; Gao, L.X. Sulfonated polyimides containing pyridine groups as proton exchange membrane materials. Chin. J. Polym. Sci. 2011, 29, 532–539. [Google Scholar] [CrossRef]
- Ghaemy, M.; Khajeh, S. Organosoluble and thermally stable polyimides derived from a new diamine containing bulky-flexible triaryl pyridine pendent group. Chin. J. Polym. Sci. 2011, 29, 465–474. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Babanzadeh, S.; Abouzari-Lotf, E. Nicotinic-based poly(amide-ether-imide)s: A new category of soluble, heat-resistant, and flame-retardant polyimides. Des. Monomers Polym. 2015, 18, 451–459. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Li, X.; Yan, S.; Yeung, K.W.K.; Chu, P.K.; Xu, Z.; Yi, C. Design and preparation of novel fluorescent polyimides containingortho-linked units and pyridine moieties. Des. Monomers Polym. 2012, 15, 389–404. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Zhang, Y.; Wang, W.; Xu, Z.; Yeung, K.W.K.; Xu, M.; Yi, C. Synthesis and characterization of highly soluble and optically transparent polyimides derived from novel fluorinated pyridine-containing aromatic diamine. High Perform. Polym. 2012, 25, 268–277. [Google Scholar] [CrossRef]
- Yan, S.Y.; Chen, W.Q.; Yan, W.; Huang, M.F.; Chen, C.; Xu, Z.S.; Yeung, K.W.K.; Yi, C.F. Optical transparency and light colour of highly soluble fluorinated polyimides derived from a novel pyridine-containing diamine m,p-3FPAPP and various aromatic dianhydrides. Des. Monomers Polym. 2011, 14, 579–592. [Google Scholar]
- Koohmareh, G.A. New organo-soluble polyimides based on a new dianhydride: 4-(4-t-butyl-phenyl)-2,6-bis(3,4-phenyl dicarboxylic acid anhydride)pyridine. Des. Monomers Polym. 2007, 10, 517–525. [Google Scholar] [CrossRef]
- Zhao, J.J.; Gong, C.L.; Zhang, S.J.; Shao, Y.; Li, Y.F. Synthesis of a new pyridine-containing diamine and related polyimide. Chin. Chem. Lett. 2010, 21, 277–278. [Google Scholar] [CrossRef]
- Sadhasivam, B.; Muthusamy, S. Thermal and dielectric properties of newly developed L-tryptophan-based optically active polyimide and its POSS nanocomposites. Des. Monomers Polym. 2016, 19, 236–247. [Google Scholar] [CrossRef]
- Mansoori, Y.; Sanaei, S.S.; Zamanloo, M.R.; Imanzadeh, G.; Atghia, S.V. Synthesis and properties of new polyimide/clay nanocomposite films. Bull. Mater. Sci. 2013, 36, 789–798. [Google Scholar] [CrossRef]
- Wang, C.B.; Guan, Y.; Tian, D.B.; Dang, G.D.; Wang, D.M.; Chen, C.H.; Zhou, H.W. Highly transparent polyimides derived from 2-phenyl-4,6-bis(4-aminophenoxy)pyrimidine and 1,3-bis(5-amino-2-pyridinoxy)benzene: preparation, characterization, and optical properties. RSC Adv. 2015, 5, 103246–103254. [Google Scholar] [CrossRef]
- Li, F.; Wan, W.J.; Lai, J.C.; Liu, F.; Qi, H.X.; Li, X.S.; You, X.Z. Investigations on the polyimides derived from unfunctionalized symmetric cyclopentyl-containing alicyclic cardo-type dianhydride. J. Appl. Polym. Sci. 2015, 132, 42670. [Google Scholar] [CrossRef]
- Bahman, T.; Gholam, A.K. Synthesis and characterization of new thermally stable poly(ether-imide)s derived from 4-aryl-2,6-bis[4-(3-nitrophthalimido)phenyl] pyridines. Des. Monomers Polym. 2007, 10, 167–180. [Google Scholar]
- An, H.Y.; Zhan, M.S.; Wang, K. Synthesis and characterization of soluble poly(ether imide)s containing fluorenyl cardo groups. J. Appl. Polym. Sci. 2009, 114, 3987–3993. [Google Scholar] [CrossRef]
- Huang, X.H.; Pei, X.L.; Wang, L.C.; Mei, M.; Liu, C.J.; Wei, C. Design and synthesis of organosoluble and transparent polyimides containing bulky substituents and noncoplanar structures. J. Appl. Polym. Sci. 2016, 133, 43266–43275. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, X.; Qi, H.; Liu, F. Synthesis of a new siloxane-containing alicyclic dianhydride and the derived polyimides with improved solubility and hydrophobicity. J. Appl. Polym. Sci. 2012, 127, 1493–1501. [Google Scholar] [CrossRef]








| Polymers | Tg (°C) a | Td (°C) b | T5% (°C) c | T10% (°C) c | Rw (%) d | Η (dL/g) | θw (°) |
|---|---|---|---|---|---|---|---|
| PI-1 | -- | 534 | 537 | 580 | 70.8 | 0.37 | 88.6 |
| PI-2 | 359 | 533 | 540 | 574 | 70.9 | 0.36 | 87.7 |
| PI-3 | 316 | 527 | 527 | 570 | 71.2 | 0.41 | 85.6 |
| PI-4 | 348 | 536 | 543 | 578 | 71.0 | 0.63 | 95.8 |
| PI-5 | 322 | 517 | 528 | 552 | 67.2 | 0.67 | 97.7 |
| Polymers | Solvent a | |||||||
|---|---|---|---|---|---|---|---|---|
| DMF b | DMAC b | DMSO b | NMP b | CHCl3 | THF b | CH2Cl2 | EA | |
| PI-1 | +− | −− | +− | + | −− | −− | −− | −− |
| PI-2 | + | +− | +− | + | −− | −− | −− | −− |
| PI-3 | ++ | ++ | ++ | ++ | + | + | −− | −− |
| PI-4 | ++ | + | + | ++ | + | +− | −− | −− |
| PI-5 | ++ | ++ | ++ | ++ | ++ | ++ | +− | −− |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, B.; Mei, M.; Li, H.; Liu, C.; Wei, C. Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine. Polymers 2017, 9, 484. https://doi.org/10.3390/polym9100484
Huang X, Chen B, Mei M, Li H, Liu C, Wei C. Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine. Polymers. 2017; 9(10):484. https://doi.org/10.3390/polym9100484
Chicago/Turabian StyleHuang, Xiaohua, Beicai Chen, Mei Mei, Hua Li, Chanjuan Liu, and Chun Wei. 2017. "Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine" Polymers 9, no. 10: 484. https://doi.org/10.3390/polym9100484
APA StyleHuang, X., Chen, B., Mei, M., Li, H., Liu, C., & Wei, C. (2017). Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy)phenyl)pyridine. Polymers, 9(10), 484. https://doi.org/10.3390/polym9100484