Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers
Abstract
:1. Introduction
2. The Role of l-Cysteine Building Blocks in Proteins and Peptides
3. Poly(Cysteine) Synthesis
3.1. Historical Overview
3.2. Synthesis of Poly(Cysteine) and Other Poly(Amino Acid)s by Controlled Ring Opening Polymerization of Amino Acid N-Carboxyanhydrides
3.3. The Role of Urea in the Synthesis of Poly(Amino Acid)s
3.4. General Synthetic Approach for the Synthesis of Poly(l-Cysteine) at Room Temperature and in the Presence of Urea
4. Poly(Cysteine) Applications
4.1. Poly(Cysteine) in Biomedical Applications
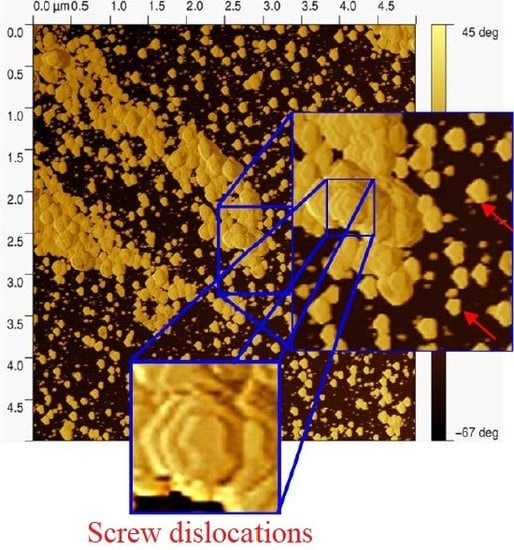
4.2. Poly(l-Cysteine) in Surface Modifications
4.3. Poly(l-Cysteine) in Drug Delivery Vehicles
4.4. The Use of Poly(l-Cysteine) in Electrochemistry as Detection Reagent
5. Conclusions
Author Contributions
Conflicts of Interest
References
- MacDonald, M. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 1993, 72, 971–983. [Google Scholar] [CrossRef]
- Scherzinger, E.; Lurz, R.; Turmaine, M.; Mangiarini, L.; Hollenbach, B.; Hasenbank, R.; Bates, G.P.; Davies, S.W.; Lehrach, H.; Wanker, E.E. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 1997, 90, 549–558. [Google Scholar] [CrossRef]
- Brais, B. Oculopharyngeal muscular dystrophy: A late-onset polyalanine disease. Cytogenet. Genome Res. 2003, 100, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Wolfenden, R.; Andersson, L.; Cullis, P.M.; Southgate, C.C.B. Affinities of amino acid side chains for solvent water. Biochemistry 1981, 20, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.D.; Geselowitz, A.R.; Lesser, G.J.; Lee, R.H.; Zehfus, M.H. Hydrophobicity of amino acid residues in globular proteins. Science 1985, 229, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Janin, J. Surface and inside volumes in globular proteins. Nature 1979, 277, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Oma, Y.; Kino, Y.; Sasagawa, N.; Ishiura, S. Comparative analysis of the cytotoxicity of homopolymeric amino acids. Biochim. Biophys. Acta 2005, 1748, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Comini, M.A. Measurement and meaning of cellular thiol: Disufhide redox status. Free Radic. Res. 2016, 50, 246–271. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Viña, J.; Saez, G.T.; Wiggins, D.; Roberts, A.F.; Hems, R.; Krebs, H.A. The effect of cysteine oxidation on isolated hepatocytes. Biochem. J. 1983, 212, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Gene expression and the thiol redox state. Free Radic. Biol. Med. 1999, 27, 936–944. [Google Scholar] [CrossRef]
- Voehringer, D.W. BCL-2 and glutathione: Alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic. Biol. Med. 1999, 27, 945–950. [Google Scholar] [CrossRef]
- Dringen, R.; Kussmaul, L.; Gutterer, J.M.; Hirrlinger, J.; Hamprecht, B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J. Neurochem. 1999, 72, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta 2013, 1830, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [PubMed]
- Jones, H.W.; Lundgren, H.P. Polycystine. J. Am. Chem. Soc. 1951, 73, 5465–5466. [Google Scholar] [CrossRef]
- Leuchs, H. Ueber die Glycin-carbonsäure. Eur. J. Inorg. Chem. 1906, 39, 857–861. [Google Scholar] [CrossRef]
- Sakakibara, S.; Tani, H. Synthesis of polycysteine. Chem. Soc. Jpn. 1956, 29, 85–88. [Google Scholar] [CrossRef]
- Deming, T.J. Controlled polymerization of a-amino acid-N-carboxyanhydrides using transition metal initiators. Polym. Prepr. 1996, 37, 435–436. [Google Scholar]
- Deming, T.J. Transition metal–amine initiators for preparation of well-defined poly(γ-benzyl l-glutamate). J. Am. Chem. Soc. 1997, 119, 2759–2760. [Google Scholar] [CrossRef]
- Deming, T.J. Amino acid derived nickelacycles: Intermediates in nickel-mediated polypeptide synthesis. J. Am. Chem. Soc. 1998, 120, 4240–4241. [Google Scholar] [CrossRef]
- Aliferis, T.; Iatrou, H.; Hadjichristidis, N. Living polypeptides. Biomacromolecules 2004, 5, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R. Polypeptides and 100 years of chemistry of alpha-amino acid N-carboxyanhydrides. Angew. Chem. Int. Ed. Engl. 2006, 45, 5752–5784. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Schlaad, H. Synthesis of nearly monodisperse polystyrene-polypeptide block copolymers via polymerisation of N-carboxyanhydrides. Chem. Commun. (Camb.) 2003, 23, 2944–2945. [Google Scholar] [CrossRef]
- Vayaboury, W.; Giani, O.; Cottet, H.; Deratani, A.; Schué, F. Living polymerization of α-amino acid N-carboxyanhydrides (NCA) upon decreasing the reaction temperature. Macromol. Rapid Commun. 2004, 25, 1221–1224. [Google Scholar] [CrossRef]
- Ulkoski, D.; Armstrong, T.; Scholz, C. Investigating the secondary structure of oligomeric poly(amino acid)s. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; Scholz, C., Kressler, J., Eds.; Oxford University Press: Washington, DC, USA, 2013; pp. 69–85. [Google Scholar]
- Blout, E.R.; de Lozé, C.; Bloom, S.M.; Fasman, G.D. The dependence of the conformations of synthetic poolypeptides on amino acid composition. J. Am. Chem. Soc. 1960, 82, 3787–3789. [Google Scholar] [CrossRef]
- Chen, T.; Ferris, R.; Zhang, J.; Ducker, R.; Zauscher, S. Stimulus-responsive polymer brushes on surfaces: Transduction mechanisms and applications. Prog. Polym. Sci. 2010, 35, 94–112. [Google Scholar] [CrossRef]
- Obeid, R.; Scholz, C. Synthesis and self-assembly of well-defined poly(amino acid) end-capped poly(ethylene glycol) and poly(2-methyl-2-oxazoline). Biomacromolecules 2011, 12, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Ulkoski, D.; Meister, A.; Busse, K.; Kressler, J.; Scholz, C. Synthesis and structure formation of block copolymers of poly(ethylene glycol) with homopolymers and copolymers of l-glutamic acid γ-benzyl ester and l-leucine in water. Colloid Polym. Sci. 2015, 293, 2147–2155. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis, 5th ed.; Wuts, P.G.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Iijima, M.; Ulkoski, D.; Sakuma, S.; Matsukuma, D.; Nishiyama, N.; Otsuka, H.; Scholz, C. Synthesis of PEGylated poly(amino acid) pentablock copolymers and their self-assembly. Polym. Int. 2016, 65, 1132–1141. [Google Scholar] [CrossRef]
- Holowka, E.P.; Pochan, D.J.; Deming, T.J. Charged polypeptide vesicles with controllable diameter. J. Am. Chem. Soc. 2005, 127, 12423–12428. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Armstrong, T.; Peng, X.; Busse, K.; Kressler, J.; Scholz, C. The behavior of poly(amino acids) containing l -cysteine and their block copolymers with poly(ethylene glycol) on gold surfaces. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 248–257. [Google Scholar] [CrossRef]
- Holmberg, K.; Bergström, K.; Brink, C.; Österberg, E.; Tiberg, F.; Harris, J.M. Effects on protein adsorption, bacterial adhesion and contact angle of grafting PEG chains to polystyrene. J. Adhes. Sci. Technol. 1993, 7, 503–517. [Google Scholar] [CrossRef]
- Ista, L.K.; Fan, H.; Baca, O.; López, G.P. Attachment of bacteria to model solid surfaces: Oligo(ethylene glycol) surfaces inhibit bacterial attachment. FEMS Microbiol. Lett. 1996, 142, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Suwa, S.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly (ethylene oxide-aspartate) block copolymer-adriamycin conjugates. J. Control. Release 1994, 29, 17–23. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Andrade, J. Blood compatibility of polyethylene oxide surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Desai, N.P.; Hubbell, J.A. Solution technique to incorporate polyethylene oxide and other water-soluble polymers into surfaces of polymeric biomaterials. Biomaterials 1991, 12, 144–153. [Google Scholar] [CrossRef]
- Desai, N.P.; Hubbell, J.A. Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J. Biomed. Mater. Res. 1991, 25, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.J.; Busse, K.; Kressler, J.; Scholz, C. Contact angle, WAXS, and SAXS analysis of poly(beta-hydroxybutyrate) and poly(ethylene glycol) block copolymers obtained via Azotobacter vinelandii UWD. Biotechnol. Prog. 2005, 21, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Hoshino, Y.; Tamura, A.; Yoshimoto, K.; Kojima, S.; Yamashita, K.; Yamanaka, I.; Otsuka, H.; Kataoka, K.; Nagasaki, Y. Creation of a mixed poly(ethylene glycol) tethered-chain surface for preventing the nonspecific adsorption of proteins and peptides. Biointerphases 2007, 2, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Eurell, T.; Gunawan, R.; Leckband, D. Chain-length dependence of the protein and cell resistance of oligo(ethylene glycol)-terminated self-assembled monolayers on gold. J. Biomed. Mater. Res. 2001, 56, 406–416. [Google Scholar] [CrossRef]
- Jorsch, C.; Schmidt, U.; Ulkoski, D.; Scholz, C.; Guenther, M.; Gerlach, G. Implantable biomedical sensor array with biocompatible hermetic encapsulation. J. Sens. Sens. Syst. 2016, 5, 229–235. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Avula, M.N.; Cho, S.H.; Xie, X.; Devener, B.V.; Solzbacher, F.; Tathireddy, P.; Magda, J.J.; et al. Smart hydrogel-based biochemical microsensor array for medical diagnostics. Adv. Sci. Technol. 2012, 85, 47–52. [Google Scholar] [CrossRef]
- Obeid, R.; Armstrong, T.; Ulkoski, D.; Peng, X.; Kressler, J.; Scholz, C. PEGylated poly(amino acid) block copolymers for surface modification and self-assembly: Synthesis and characterization. Polym. Prepr. 2012, 53, 334–335. [Google Scholar]
- Keith, H.D.; Chen, W.Y. On the origins of giant screw dislocations in polymer lamellae. Polymer 2002, 43, 6263–6272. [Google Scholar] [CrossRef]
- Liu, G.; Dong, C. Photoresponsive poly(s-(o-nitrobenzyl)-l-cysteine)-b-PEO from a l-Cysteine N-carboxyanhydride monomer: Synthesis, self-assembly, and phototriggered drug release. Biomacromolecules 2012, 13, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, L.; Su, Y.; Dong, C.-M. Comb-like poly(l-cysteine) derivatives with different side groups: Synthesis via photochemistry and click chemistry, multi-responsive nanostructures, triggered drug release and cytotoxicity. Polym. Chem. 2015, 6, 6857–6869. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, L.; Guan, Y.; Su, Y.; Dong, C.M. Multi-responsive polypeptidosome: Characterization, morphology transformation, and triggered drug delivery. Macromol. Rapid Commun. 2014, 35, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Nishiyama, N.; Kataoka, K. Supramolecular nanodevices: From design validation to theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, L.; Su, Y.; Dong, C.M. Plasmonic, targeted, and dual drugs-loaded polypeptide composite nanoparticles for synergistic cocktail chemotherapy with photothermal therapy. Biomacromolecules 2016, 17, 2489–2501. [Google Scholar] [CrossRef] [PubMed]
- Sulistio, A.; Blencowe, A.; Widjaya, A.; Zhang, X.; Qiao, G. Development of functional amino acid-based star polymers. Polym. Chem. 2012, 3, 224–234. [Google Scholar] [CrossRef]
- Blencowe, A.; Tan, J.F.; Goh, T.K.; Qiao, G.G. Core cross-linked star polymers via controlled radical polymerisation. Polymer 2009, 50, 5–32. [Google Scholar] [CrossRef]
- Borase, T.; Iacono, M.; Ali, S.I.; Thornton, P.D.; Heise, A. Polypeptide core–shell silica nanoparticles with high grafting density by N-carboxyanhydride (NCA) ring opening polymerization as responsive materials and for bioconjugation. Polym. Chem. 2012, 3, 1267–1275. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Fu, W.; Li, Z. Thermoresponsive Oligo(ethylene glycol) functionalized poly-l-cysteine. Macromolecules 2013, 46, 3753–3760. [Google Scholar] [CrossRef]
- Fu, X.; Ma, Y.; Shen, Y.; Fu, W.; Li, Z. Oxidation-Responsive OEGylated poly-l-cysteine and solution properties studies. Biomacromolecules 2014, 15, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Tatemichi, M.; Sawa, T. Chemical basis of inflammation-induced carcinogenesis. Arch. Biochem. Biophys. 2003, 417, 3–11. [Google Scholar] [CrossRef]
- Yi, H.; Liu, P.; Sheng, N.; Gong, P.; Ma, Y.; Cai, L. In Situ crosslinked smart polypeptide nanoparticles for multistage responsive tumor-targeted drug delivery. Nanoscale 2016, 8, 5985–5995. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, X.; Lu, T.; Liu, S.; Tian, H.; Guo, Z.; Jing, X. Formation of reversible shell cross-linked micelles from the biodegradable amphiphilic diblock copolymer poly(l-cysteine)-block-poly(l-lactide). Langmuir 2008, 24, 10099–10106. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Kim, I.; Batt, C.A. A facile preparative method for aggregation-free gold nanoparticles using poly (styrene-block-cysteine). Angew. Chem. Int. Ed. 2007, 46, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, V.A.; Lee, M.C.; Bae, Y.H. A biodegradable pH-sensitive micelle system for targeting acidic solid tumors. Pharm. Res. 2008, 25, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Autry, H.A.; Holcombe, J.A. Cadmium, copper and zinc complexes of poly-l-cysteine. Analyst 1995, 120, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Jurbergs, H.A.; Holcombe, J.A. Characterization of Immobilized Poly(l-cysteine) for cadmium chelation and preconcentration. Anal. Chem. 1997, 69, 1893–1898. [Google Scholar] [CrossRef]
- Howard, M.; Jurbergs, H.A.; Holcombe, J.A. Effects of oxidation of immobilized poly(l-cysteine) on trace metal chelation and preconcentration. Anal. Chem. 1998, 70, 1604–1609. [Google Scholar] [CrossRef]
- Howard, M.; Jurbergs, H.A.; Holcombe, J.A. Comparison of silica-immobilized poly(l-cysteine) and 8-hydroxyquinoline for trace metal extraction and recovery. J. Anal. At. Spectrom. 1999, 14, 1209–1214. [Google Scholar] [CrossRef]
- Miller, T.C.; Kwak, E.S.; Howard, M.E.; Vanden Bout, D.A.; Holcombe, J.A. An in situ study of metal complexation by an immobilized synthetic biopolymer using tapping mode liquid cell atomic force microscopy. Anal. Chem. 2001, 73, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.C.; Holcombe, J.A. Characterization of metal ion-exchange on modified surfaces of porous carbon. Anal. Chim. Acta 2002, 455, 233–244. [Google Scholar] [CrossRef]
- White, B.R.; Stackhouse, B.T.; Holcombe, J.A. Magnetic gamma-Fe(2)O(3) nanoparticles coated with poly-l-cysteine for chelation of As(III), Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). J. Hazard. Mater. 2009, 161, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Holcombe, J.A. Poly(l-cysteine) as an electrochemically modifiable ligand for trace metal chelation. Anal. Chem. 2005, 77, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, D.; Xiang, D.; Huang, W.; Lu, S. Electrochemical determination of ascorbic acid using the poly-cysteine film-modified electrode. Russ. J. Electrochem. 2009, 45, 1183–1187. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Liu, G.; Sun, D. Determination of sunset yellow and tartrazine using silver and poly (l-cysteine) composite film modified glassy carbon electrode. Indian J. Chem. 2016, 55A, 298–303. [Google Scholar]
- Wang, J.; Shi, Z.; Jing, J.; Tan, Y.; Zhang, S.; Tian, D. Simultaneous determination of hydroquinone and catechol based on three-dimensionally ordered macroporous polycysteine film modified electrode. Russ. J. Electrochem. 2016, 52, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Jin, J.; Liu, Q.; Zhang, S. Determination of 4-aminophenol using a glassy carbon electrode modified with a three-dimensionally ordered macroporous film of polycysteine. Microchim. Acta 2015, 182, 823–829. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Z.; Wang, J.; Cheng, Q.; Wu, K. Preparation of three-dimensionally ordered macroporous polycysteine film and application in sensitive detection of 4-chlorophenol. Electrochim. Acta 2014, 130, 734–739. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, W.; Chen, R.; Zhang, L.; Zhang, Z. β-Cyclodextrin-Functionalized gold nanoparticles/poly(l-cysteine) modified glassy carbon electrode for sensitive determination of metronidazole. Electroanalysis 2013, 25, 1209–1216. [Google Scholar] [CrossRef]
- Wang, Z. Electrochemical investigation of methyl parathion at a poly-l-cysteine film-modified glassy carbon electrode. Chem. Anal. 2009, 25, 1209–1216. [Google Scholar]
- Wang, L.; Huang, P.; Bai, J.; Wu, X.; Wang, H.; Zhao, Y. Simultaneous determination of hydroquinone and catechol in binary mixtures using poly(cysteine)-modified glassy carbon electrode. Chem. Anal. 2007, 52, 15–24. [Google Scholar]
- Ma, W.; Yao, X.; Sun, D. Simultaneous electrochemical determination of dopamine, epinephrine and uric acid at silver doped poly-l-cysteine film electrode. Asian J. Chem. 2013, 25, 6625–6634. [Google Scholar]
- Ferraz, B.R.L.; Leite, F.R.F.; Malagutti, A.R. Simultaneous determination of ethionamide and pyrazinamide using poly(l-cysteine) film-modified glassy carbon electrode. Talanta 2016, 154, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luo, K.; Liu, G.; Hou, X.; Huang, Y.; Li, C. Voltammetric determination of butyl hydroxy anisd based on the enhanced effect of gold nanorods assembled on a poly-l-cysteine film electrode. Int. J. Electrochem. Sci. 2016, 11, 1327–1336. [Google Scholar]
- Wang, Y.; Liu, G.; Hou, X.; Huang, Y.; Li, C.; Wu, K. Assembling gold nanorods on a poly-cysteine modified glassy carbon electrode strongly enhance the electrochemical reponse to tetrabromobisphenol A. Microchim. Acta 2016, 183, 689–696. [Google Scholar] [CrossRef]
- Ma, W. Preparation of silver-doped poly(l-cysteine acid) modified electrode and its determination of uric acid. Asian J. Chem. 2011, 23, 5331–5334. [Google Scholar]
- Babu, R.S.; Prabhu, P.; Narayanan, S.S. Enzyme-free selective determination of H2O2 and glucose using functionalized CuNP-modified graphite electrode in room temperature ionic liquid medium. RSC Adv. 2014, 4, 47497–47504. [Google Scholar] [CrossRef]
- Deinhammer, R.S.; Ho, M.; Anderegg, J.W.; Porter, M.D.; Porter, M.D.; Deinhammer, R.S.; Ho, M.; Anderegg, J.W. Electrochemical oxidation of amine-containing compounds: A route to the surface modification of glassy carbon electrodes. Langmuir 1994, 10, 1306–1313. [Google Scholar] [CrossRef]
- Mann, C.K. Cyclic Stationary electrode voltammetry of some aliphatic amines. Anal. Chem. 1964, 36, 2424–2426. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X. Covalent modification of glassy carbon electrode with glutamic acid for simultaneous determination of uric acid and ascorbic acid. Analyst 2001, 126, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, C.; Wang, F.; Wang, C. Covalent modification of glassy carbon electrode with l-cysteine for the determination of acetaminophen. Microchim. Acta 2006, 155, 365–371. [Google Scholar] [CrossRef]
- Pchelintsev, N.A.; Vakurov, A.; Hays, H.H.; Millner, P.A. Thiols deposition onto the surface of glassy carbon electrodes mediated by electrical potential. Electrochim. Acta 2011, 56, 2696–2702. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulkoski, D.; Scholz, C. Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers. Polymers 2017, 9, 500. https://doi.org/10.3390/polym9100500
Ulkoski D, Scholz C. Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers. Polymers. 2017; 9(10):500. https://doi.org/10.3390/polym9100500
Chicago/Turabian StyleUlkoski, David, and Carmen Scholz. 2017. "Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers" Polymers 9, no. 10: 500. https://doi.org/10.3390/polym9100500
APA StyleUlkoski, D., & Scholz, C. (2017). Synthesis and Application of Aurophilic Poly(Cysteine) and Poly(Cysteine)-Containing Copolymers. Polymers, 9(10), 500. https://doi.org/10.3390/polym9100500