Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Polymer Films Doped with Au Nanoparticle Aggregates
2.2. Characterization Techniques
2.3. Measurement of Fractal Dimension
2.4. SERS Experiments
3. Results and Discussion
3.1. Generation of Au Nanoparticles in the Epoxy Medium
3.2. Flexible, Free-Standing Thin Films Embedded with Fractal Aggregates of Au Nanoparticles
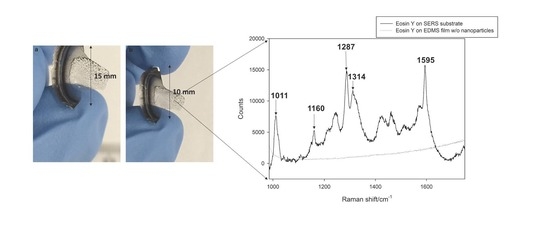
3.3. Surface Enhanced Raman Scattering
4. Summary and Outlook
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raman, C.V.; Krishnan, S.K. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- McCreery, R.L. Raman Spectroscopy for Chemical Analysis; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single Molecule Detection Using Surface-Enhanced Raman Scattering ( SERS ). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.D.; Schwartzberg, A.M.; Norman, T.J.; Zhang, J.Z. Ultrafast electronic relaxation and coherent vibrational oscillation of strongly coupled gold nanoparticle aggregates. J. Am. Chem. Soc. 2003, 125, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Cheng, L.; Lee, S.; Liu, Z. Noble Metal Coated Single-Walled Carbon Nanotubes for Applications in Surface Enhanced Raman Scattering Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Link, S.; Halas, N.J. Nano-optics from sensing to waveguiding. Nat. Photonics 2007, 1, 641–648. [Google Scholar] [CrossRef]
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured surfaces and assemblies as SERS media. Small 2008, 4, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Kong, X. Review of Recent Progress of Plasmonic Materials and Nano-Structures for Surface-Enhanced Raman Scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.-B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanooctahedra with Tunable Size and Microfluidic-Induced 3D Assembly for Highly Uniform SERS-Active Supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Liu, T.-Y.; Su, Y.-A.; Wu, C.-H.; Cheng, Y.-W.; Cheng, H.-W.; Jeng, R.-J. Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers 2017, 9, 93. [Google Scholar] [CrossRef]
- Fernández-López, C.; Polavarapu, L.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Contreras-Cáceres, R.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanorod–pNIPAM Hybrids with Reversible Plasmon Coupling: Synthesis, Modeling, and SERS Properties. ACS Appl. Mater. Interfaces 2015, 7, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.Y.; Xu, H.; Natan, M.J.; Cromer, R. Surface-Enhanced Raman Scattering Tags for Rapid and Homogeneous Detection of Circulating Tumor Cells in the Presence of Human Whole Blood. J. Am. Chem. Soc. 2008, 130, 17214–17215. [Google Scholar] [CrossRef] [PubMed]
- Piorek, B.D.; Lee, S.J.; Santiago, J.G.; Moskovits, M.; Banerjee, S.; Meinhart, C.D. Free-surface microfluidic control of surface-enhanced Raman spectroscopy for the optimized detection of airborne molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 18898–18901. [Google Scholar] [CrossRef] [PubMed]
- Monolayers, S.; Paxton, W.F.; Kleinman, S.L.; Basuray, A.N.; Stoddart, J.F.; Duyne, R.P. Van Surface-Enhanced Raman Spectroelectrochemistry of TTF-Modified. J. Phys. Chem. Lett. 2011, 2, 1145–1149. [Google Scholar]
- Kleinman, S.L.; Ringe, E.; Valley, N.; Wustholz, K.L.; Phillips, E.; Scheidt, K.A.; Schatz, G.C.; Duyne, R.P. Van Single-Molecule Surface-Enhanced Raman Spectroscopy of Crystal Violet Isotopologues: Theory and Experiment. J. Am. Chem. Soc. 2011, 133, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Leona, M. Microanalysis of organic pigments and glazes in polychrome works of art by surface-enhanced resonance Raman scattering. Proc. Natl. Acad. Sci. USA 2009, 106, 14757–14762. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.R.; White, D.J. Optical fibre SERS sensors. Anal. Bioanal. Chem. 2009, 394, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.; Ringe, E.; Duyne, R.P. Van SERS: Materials, applications, and the future Surface enhanced Raman spectroscopy (SERS) is a powerful vibrational. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Knoll, B.; Keilmann, F. Near- field probing of vibrational absorption for chemical microscopy. Nature 1999, 399, 134–136. [Google Scholar] [CrossRef]
- Pozzi, E.A.; Zrimsek, A.B.; Lethiec, C.M.; Schatz, G.C.; Hersam, M.C.; Duyne, R.P. Van Evaluating Single-Molecule Stokes and Anti-Stokes SERS for Nanoscale Thermometry. J. Phys. Chem. C 2015, 119, 21116–21124. [Google Scholar] [CrossRef]
- Michaels, A.M.; Brus, L. Ag Nanocrystal Junctions as the Site for Surface-Enhanced Raman Scattering of Single Rhodamine 6G Molecules. J. Phys. Chem. B 2000, 104, 11965–11971. [Google Scholar] [CrossRef]
- Xu, H.; Bjerneld, E.; Käll, M.; Börjesson, L. Spectroscopy of Single Hemoglobin Molecules by Surface Enhanced Raman Scattering. Phys. Rev. Lett. 1999, 83, 4357–4360. [Google Scholar] [CrossRef]
- Stockman, M.I.; Shalaev, V.M.; Moskovits, M.; Botet, R.; George, T.F. Enhanced Raman scattering by fractal clusters: Scale-invariant theory. Phys. Rev. B 1992, 46, 2821–2831. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Surface-Enhanced Raman Scattering Studies on Aggregated Gold Nanorods †. J. Phys. Chem. A 2003, 107, 3372–3378. [Google Scholar] [CrossRef]
- Faulds, K.; Littleford, R.E.; Graham, D.; Dent, G.; Smith, W.E. Comparaison of surface enhanced resonance raman scattering from unaggregated anf aggregated nanoparticles. Anal. Chem. 2004, 76, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Shalaev, V.M.; Stockman, M.I. Fractals: Optical susceptibility and giant raman scattering. Z. Phys. D Atoms Mol. Clust. 1988, 10, 71–79. [Google Scholar] [CrossRef]
- Sanchez-Gil, J.A.; Garcia-Ramos, J.A. Calculations of the direct electromagnetic enhancement in surface enhanced Raman scattering on random self-affine fractal metal surfaces. J. Chem. Phys. 1998, 108, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Pan, S.; Krauss, T.D.; Du, H.; Rothberg, L.J. The structural basis for giant enhancement enabling single-molecule Raman scattering. Proc. Natl. Acad. Sci. USA 2003, 100, 8638–8643. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Stockman, M.I.; Bergman, D.J. Self-Similar Chain of Metal Nanospheres as an Efficient Nanolens. Phys. Rev. Lett. 2003, 91, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, X.L.; Shen, J.C.; Xia, Y.; Shen, P.N.; Chu, P.K. Applied Surface Science Silver fractal networks for surface-enhanced Raman scattering substrates. Appl. Surf. Sci. 2008, 254, 5399–5402. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S. Designing Fractal Nanostructured Biointerfaces for Biomedical Applications. ChemPhysChem 2014, 15, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, Y.; Wing, M.; Mak, C.; Cheung, K.Y.; Li, X.; Renneberg, R.; Yang, S. Dendritic Nanostructures of Silver: Facile Synthesis, Structural Characterizations, and Sensing Applications. Langmuir 2006, 22, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qu, S.; Zheng, H.; Zhang, Z.; Li, J.; Huo, Y.; Li, G. Sensors and Actuators B: Chemical Simultaneous SEF and SERRS from silver fractal-like nanostructure. Sens. Actuators B 2014, 191, 595–599. [Google Scholar] [CrossRef]
- Kim, W.; Safonov, V.P.; Shalaev, V.M.; Armstrong, R.L. Fractals in Microcavities: Giant Coupled, Multiplicative Enhancement of Optical Responses. Phys. Rev. Lett. 1999, 82, 4811–4814. [Google Scholar] [CrossRef]
- He, L.; Kim, N.K.; Li, H.; Hu, Z.; Lin, M. Use of a Fractal-like Gold Nanostructure in Surface-Enhanced Raman Spectroscopy for detection of selected food contaminants. J. Agric. Food Chem. 2008, 56, 9843–9847. [Google Scholar] [CrossRef] [PubMed]
- Amarandei, G.; Arshak, A.; Corcoran, D. Fractal Patterning of Nanoparticles on Polymer Films and Their SERS Capabilities. Appl. Mater. Interfaces 2013, 5, 8655–8662. [Google Scholar] [CrossRef] [PubMed]
- Shalaev, V.A.; Shalaev, V.M.; Stechel, E.B.; Kim, W.; Armstrong, R.L. Small-particle composites. I. Linear Optical Properties. Phys. Rev. B 1996, 53, 2425–2436. [Google Scholar] [CrossRef]
- Shalaev, V.M. Electromagnetic properties of small-particle composites. Phys. Rep. 1996, 272, 61–137. [Google Scholar] [CrossRef]
- Brouers, F.; Blacher, S.; Sarychev, A.K. Giant field fluctuations and anomalous light scattering from semicontinuous metal films. Phys. Rev. B 1998, 58, 897–903. [Google Scholar] [CrossRef]
- Chen, T.L.; Dikken, D.J.; Prangsma, J.C.; Segerink, F.; Herek, J.L. Characterization of Sierpinski carpet optical antenna at visible and near-infrared wavelengths. New J. Phys. 2014, 16, 93024. [Google Scholar] [CrossRef]
- Liu, J.; Fu, Y.; Guo, A.; Wang, C.; Huang, R.; Zhang, X. Growth of Gold Fractal Nanostructures by Electrochemical Deposition in Organic Electrolytes: Morphologies and Their Transitions. J. Phys. Chem. C 2008, 112, 4242–4247. [Google Scholar] [CrossRef]
- Cavaliere, E.; Ferrini, G.; Pingue, P.; Gavioli, L. Fractal TiO2 Nanostructures by Nonthermal Laser Ablation at Ambient Pressure. J. Phys. Chem. C 2013, 117, 23305–23312. [Google Scholar] [CrossRef]
- Agrawal, V.V.; Kulkarni, G.U.; Rao, C.N.R. Surfactant-promoted formation of fractal and dendritic nanostructures of gold and silver at the organic—Aqueous interface. J. Colloid Interface Sci. 2008, 318, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gong, J.; Liu, J.; Chen, M.; Yan, X. Electrochimica Acta The decoration of silver fractal-like nanostructure with Ag nanoparticles on the plastic slide for surface enhanced fluorescence. Electrochim. Acta 2012, 60, 264–268. [Google Scholar] [CrossRef]
- Gottheim, S.; Zhang, H.; Govorov, A.O.; Halas, N.J. Fractal Nanoparticle Plasmonics: The Cayley Tree. ACS Nano 2015, 9, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Brouers, F.; Blacher, S.; Lagarkov, A.N.; Sarychev, A.K.; Gadenne, P.; Shalaev, V.M. Theory of giant Raman scattering from semicontinuous metal films. Phys. Rev. B 1997, 55, 234–245. [Google Scholar] [CrossRef]
- Liu, R.; Xiong, Y.; Tang, W.; Guo, Y.; Yan, X.; Si, M. Near-infrared surface-enhanced Raman spectroscopy (NIR-SERS) studies on oxyheamoglobin (OxyHb) of liver cancer based on PVA-Ag nanofilm. J. Raman Spectrosc. 2013, 44, 362–369. [Google Scholar] [CrossRef]
- Samanta, A.; Maiti, K.K.; Soh, K.S.; Liao, X.; Vendrell, M.; Dinish, U.S.; Yun, S.W.; Bhuvaneswari, R.; Kim, H.; Rautela, S.; et al. Ultrasensitive near-infrared Raman reporters for SERS-based in vivo cancer detection. Angew. Chem. Int. Ed. 2011, 50, 6089–6092. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Centrone, A.; Park, J.H.; Ramanathan, R.; Sailor, M.J.; Alan Hatton, T.; Bhatia, S.N. SERS-coded cold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv. Mater. 2009, 21, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Cakmakyapan, S.; Cinel, N.A.; Cakmak, A.O.; Ozbay, E. Validation of electromagnetic field enhancement in near-infrared through Sierpinski fractal nanoantennas. Opt. Express 2014, 22, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.M.; Campione, S.; Caldwell, J.D.; Bezares, F.J.; Culbertson, J.C.; Capolino, F.; Ragan, R. Non-lithographic SERS Substrates: Tailoring Surface Chemistry for Au Nanoparticle Cluster Assembly. Small 2012, 8, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.V.; Herzog, G. In situ observation of cluster formation during nanoparticle solution casting on a colloidal film. J. Phys. Condens. Matters 2011, 23, 254208–254217. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent Vapor Annealing of Block Polymer Thin Films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Gebhardt, R.; Burghammer, M.; Riekel, C.; Roth, V.; Mu, P. Structural Changes of Casein Micelles in a Calcium Gradient Film. Macromol. Biosci. 2008, 8, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.; Commiskey, P.; Lazaridis, F.; Argyrakis, P.; Kopelman, R. Fractal Dimension of Microbead Assemblies Used for Protein Detection. Chem. Phys. Chem. 2014, 15, 3444–3446. [Google Scholar] [CrossRef] [PubMed]
- Ethers, P.P. Polymeric Pseudocrown Ethers. 1. Synthesis and Complexation with Transition Metal Anions. J. Am. Chem. Soc. 1979, 101, 4249–4258. [Google Scholar]
- Longenberger, L.; Mills, G. Formation of Metal Particles in Aqueous Solutions by Reactions of Metal Complexes with polymers. J. Phys. Chem. 1995, 99, 475–478. [Google Scholar] [CrossRef]
- Ishii, T.; Otsuka, H.; Kataoka, K.; Nagasaki, Y. Preparation of Functionally PEGylated Gold Nanoparticles with Narrow Distribution through Autoreduction of Auric Cation by α-Biotinyl-PEG-block-[poly(2-(N,N-dimethylamino)ethyl methacrylate)]. Langmuir 2004, 20, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of Metal Nanoparticles: The influence of seze, shape and dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Pong, B.K.; Elim, H.I.; Chong, J.X.; Ji, W.; Trout, B.L.; Lee, J.Y. New insights on the nanoparticle growth mechanism in the citrate reduction of gold(III) salt: Formation of the Au nanowire intermediate and its nonlinear optical properties. J. Phys. Chem. C 2007, 111, 6281–6287. [Google Scholar] [CrossRef]
- Witten, T., Jr.; Sander, L. Diffusion-Limited Aggregation, a Kinetic Critical Phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Wang, S.; Xin, H. Fractal and Dendritic Growth of Metallic Ag Aggregated from Different Kinds of γ-Irradiated Solutions. J. Phys. Chem. B 2000, 104, 5681–5685. [Google Scholar] [CrossRef]
- Halsey, T.C. Diffusion-Limited Aggregation: A Model for Pattern Formation. Phys. Today 2000, 53, 36. [Google Scholar] [CrossRef]
- Enculescu, M.; Enculescu, I.; Topa, V. Fractal structures of gold obtained by diffusion limited aggregation in alkali halide crystals. J. Optoelectron. Adv. Mater. 2006, 8, 1230–1233. [Google Scholar]
- Cheng, W.; Dong, S.; Wang, E. Spontaneous fractal aggregation of gold nanoparticles and controlled generation of aggregate-based fractal networks at air/water interface. J. Phys. Chem. B 2005, 109, 19213–19218. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wei, Q.; Zhang, X. Interparticle Coupling Effects on Plasmon Resonances of Nanogold Particles. Nano Lett. 2003, 3, 1087–1090. [Google Scholar] [CrossRef]
- Jensen, T.; Kelly, L.; Lazarides, A.; Schatz, G.C. Electrodynamics of noble metal nanoparticles and nanoparticle clusters. J. Clust. Sci. 1999, 10, 295–317. [Google Scholar] [CrossRef]
- Zhong, Z.; Patskovskyy, S.; Bouvrette, P.; Luong, J.H.T.; Gedanken, A. The Surface Chemistry of Au Colloids and Their Interactions with Functional Amino Acids. J. Phys. Chem. B 2004, 108, 4046–4052. [Google Scholar] [CrossRef]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold nanoparticles aggregation: Drastic effect of cooperative functionalities in a single molecular conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic Polymerization—Iodonium and Sulfonium Salt Photoinitiators. Adv. Polym. Sci. 1984, 62, 1–48. [Google Scholar]
- Walker, J.M. The Protein Protocols Handbook, 2nd ed.; Humana Press Inc.: Totowa, NJ, USA, 2002; Volume 3, ISBN 0896039404. [Google Scholar]
- Greeneltch, N.G.; Davis, A.S.; Valley, N.A.; Casadio, F.; Schatz, G.C.; Van Duyne, R.P.; Shah, N.C. Near-infrared surface-enhanced raman spectroscopy (NIR-SERS) for the identification of eosin Y: Theoretical calculations and evaluation of two different nanoplasmonic substrates. J. Phys. Chem. A 2012, 116, 11863–11869. [Google Scholar] [CrossRef] [PubMed]
- Payne, E.K.; Rosi, N.L.; Xue, C.; Mirkin, C.A. Sacrificial biological templates for the formation of nanostructured metallic microshells. Angew. Chem. Int. Ed. 2005, 44, 5064–5067. [Google Scholar] [CrossRef] [PubMed]
- Takahiro, G.; Itagaki, S.; Kajiwara, T.; Abe, Y.; Hatakeyama, T.; Aoki, R. Preparation and Properties of Siloxane/Epoxy Organic-Inorganic Hybrid Thin Films, Self-Standing Films, and Bulk Bodies. Polym. J. 2009, 41, 541–546. [Google Scholar] [CrossRef]






| Shifted Calculated Mode cm−1 | Description | Experimental Shift cm−1 |
|---|---|---|
| 1003 | xanthene ring breathing + C−Br stretches | 1011 |
| 1158 | xanthene and benzene ring stretches and symmetric CO2 stretch | 1160 |
| 1260 | xanthene and benzene ring C−C stretches | 1287 |
| 1310 | xanthene and benzene ring stretches and symmetric CO2 stretch | 1314 |
| 1579 | xanthene and benzene ring stretches and symmetric CO2 stretch | 1579 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basker, D.K.; Saravanamuttu, K. Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy. Polymers 2017, 9, 507. https://doi.org/10.3390/polym9100507
Basker DK, Saravanamuttu K. Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy. Polymers. 2017; 9(10):507. https://doi.org/10.3390/polym9100507
Chicago/Turabian StyleBasker, Dinesh K., and Kalaichelvi Saravanamuttu. 2017. "Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy" Polymers 9, no. 10: 507. https://doi.org/10.3390/polym9100507
APA StyleBasker, D. K., & Saravanamuttu, K. (2017). Spontaneous Formation of Fractal Aggregates of Au Nanoparticles in Epoxy-Siloxane Films and Their Application as Substrates for NIR Surface Enhanced Raman Spectroscopy. Polymers, 9(10), 507. https://doi.org/10.3390/polym9100507