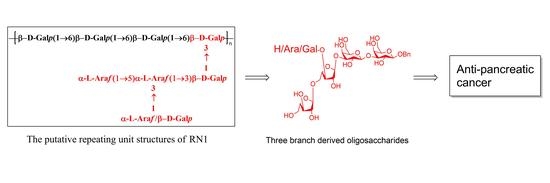
A Concise Synthesis of Three Branches Derived from Polysaccharide RN1 and Anti-Pancreatic Cancer Activity Study
Abstract
:1. Introduction
2. Materials and Method
2.1. General Experimental Procedures
2.2. Cells and Materials
2.3. Cell Viability Assessment
3. Result and Discussion
3.1. Retrosynthetic Analysis
3.2. Synthesis of Donors 7, 9, and Acceptor 14
3.3. Exploration of the Synthesis of the Gal-Containing Trisaccharide Donor
3.4. Synthesis of Target Compound 18, 21
3.5. Synthesis of the Target and Challenging 27
3.6. Anti-Pancreatic Cancer Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Wang, Y.; Kuramitsu, Y.; Kitagawa, T.; Tokuda, K.; Baron, B.; Akada, J.; Nakamura, K. The histone deacetylase inhibitor valproic acid sensitizes gemcitabine-induced cytotoxicity in gemcitabine-resistant pancreatic cancer cells possibly through inhibition of the DNA repair protein gamma-H2AX. Target. Oncol. 2015, 10, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Dalla Pozza, E.; Scupoli, M.T.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef] [PubMed]
- Dastjerdi, M.N.; Babazadeh, Z.; Salehi, M.; Hashemibeni, B.; Kazemi, M. Comparison of the anti-cancer effect of disulfiram and 5-Aza-CdR on pancreatic cancer cell line PANC-1. Adv. Biomed. Res. 2014, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Bollu, R.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Jain, N.; Vangala, R.; Manga, V. Design, synthesis and docking studies of novel 1,2-dihydro-4-hydroxy-2-oxoquinoline-3-carboxamide derivatives as a potential anti-proliferative agents. Eur. J. Med. Chem. 2017, 125, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, S.B.; Kim, S.A.; Kwon, S.K.; Cha, H.; Lee, D.Y.; Ro, S.; Cho, J.M.; Song, S.Y. A novel HDAC inhibitor, cg200745, inhibits pancreatic cancer cell growth and overcomes gemcitabine resistance. Sci. Rep. 2017, 7, 41615. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.; Sahu, R.P. Natural anti-cancer agents: Implications in gemcitabine-resistant pancreatic cancer treatment. Mini Rev. Med. Chem. 2017, 17, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug. Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Yao, J.; Shi, Y.; Li, P.; Ding, K. An arabinogalactan from flowers of panax notoginseng inhibits angiogenesis by bmp2/smad/id1 signaling. Carbohydr. Polym. 2015, 121, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, P.; Qin, Y.; Cong, Q.; Shao, C.; Du, Z.; Ni, X.; Li, P.; Ding, K. Rn1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene 2017, 36, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-Y.; Deng, L.-M.; Liu, X.; Yang, J.-S. Efficient one-pot syntheses of α-d-arabinofuranosyl tri- and tetrasaccharides present in cell wall polysaccharide of mycobacterium tuberculosis. Tetrahedron 2010, 66, 87–93. [Google Scholar] [CrossRef]
- Sylla, B.; Descroix, K.; Pain, C.; Gervaise, C.; Jamois, F.; Yvin, J.C.; Legentil, L.; Nugierchauvin, C.; Daniellou, R.; Ferrières, V. Double diastereoselection explains limitations in synthesizing mannose-containing beta-(1,3)-glucans. Carbohydr. Res. 2010, 345, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ning, J.; Kong, F. Pure α-linked products can be obtained in high yields in glycosylation with glucosyl trichloroacetimidate donors with a c2 ester capable of neighboring group participation. Tetrahedron Lett. 2002, 43, 3729–3733. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hung, S.C.; Wu, C.Y.; Wong, C.H. Toward automated oligosaccharide synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 11872–11923. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-Y.; Bin, H.-C.; Yang, J.-S. Tuning effect of silyl protecting groups on the glycosylation reactivity of arabinofuranosyl thioglycosides. Org. Lett. 2013, 15, 2834–2837. [Google Scholar] [CrossRef] [PubMed]
- Almendros, M.; Danalev, D.; Francois-Heude, M.; Loyer, P.; Legentil, L.; Nugier-Chauvin, C.; Daniellou, R.; Ferrieres, V. Exploring the synthetic potency of the first furanothioglycoligase through original remote activation. Org. Biomol. Chem. 2011, 9, 8371–8378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Yoshida, K.; Yin, Z.; Dai, H.; Kavunja, H.; El-Dakdouki, M.H.; Sungsuwan, S.; Dulaney, S.B.; Huang, X. Chemical synthesis of a heparan sulfate glycopeptide: Syndecan-1. Angew. Chem. Int. Ed. Engl. 2012, 51, 10185–10189. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Yao, Y.; Tang, Y.; Wang, Z.; Shi, W.; Huang, W.; Ding, K. A Concise Synthesis of Three Branches Derived from Polysaccharide RN1 and Anti-Pancreatic Cancer Activity Study. Polymers 2017, 9, 536. https://doi.org/10.3390/polym9100536
Cai D, Yao Y, Tang Y, Wang Z, Shi W, Huang W, Ding K. A Concise Synthesis of Three Branches Derived from Polysaccharide RN1 and Anti-Pancreatic Cancer Activity Study. Polymers. 2017; 9(10):536. https://doi.org/10.3390/polym9100536
Chicago/Turabian StyleCai, Deqin, Yanli Yao, Yubo Tang, Zheng Wang, Wei Shi, Wei Huang, and Kan Ding. 2017. "A Concise Synthesis of Three Branches Derived from Polysaccharide RN1 and Anti-Pancreatic Cancer Activity Study" Polymers 9, no. 10: 536. https://doi.org/10.3390/polym9100536
APA StyleCai, D., Yao, Y., Tang, Y., Wang, Z., Shi, W., Huang, W., & Ding, K. (2017). A Concise Synthesis of Three Branches Derived from Polysaccharide RN1 and Anti-Pancreatic Cancer Activity Study. Polymers, 9(10), 536. https://doi.org/10.3390/polym9100536