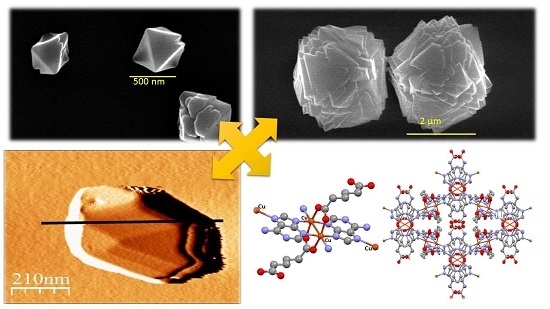
Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis
2.2.1. Synthesis of {[Cu2(μ3-adeninato)2(μ-Hadip)2]}n (1m)
2.2.2. Synthesis of Submicron {[Cu2(μ3-adeninato)2(μ-Hadip)2]}n (1n)
2.2.3. Surfactant-Mediated Syntheses of Submicron {[Cu2(μ3-adeninato)2(μ-Hadip)2]}n (1n)
3. Results and Discussion
3.1. Effect of Temperature, Reaction Time and Concentration in the Size and Shape of {[Cu2(μ3-adeninato)2(μ-Hadip)2]}n (1n)
3.2. Effect of Surfactant in the Size and Shape of {[Cu2(μ3-Adeninato)2(μ-Hadip)2]}n (1n)
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Applications; RSC Publishing: London, UK, 2009; pp. 238–256. [Google Scholar]
- Amo-Ochoa, P.; Alexandre, S.S.; Hribesh, S.; Galindo, M.A.; Castillo, O.; Gómez-García, C.J.; Pike, A.J.; Soler, J.M.; Houlton, A.; Zamora, F. Coordination Chemistry of 6-Thioguanine Derivatives with Cobalt: Toward Formation of Electrical Conductive One-Dimensional Coordination Polymers. Inorg. Chem. 2013, 52, 5290–5299. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Castillo, O.; Alexandre, S.S.; Welte, L.; de Pablo, P.J.; Rodríguez-Tapiador, M.I.; Gómez-Herrero, J.; Zamora, F. Synthesis of Designed Conductive One-Dimensional Coordination Polymers of Ni(II) with 6-Mercaptopurine and 6-Thioguanine. Inorg. Chem. 2009, 48, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Zamora, F. Coordination polymers with nucleobases: From structural aspects to potential applications. Coord. Chem. Rev. 2014, 276, 34–58. [Google Scholar] [CrossRef]
- Zhang, W.X.; Shiga, T.; Miyasaka, H.; Yamashita, M. New Approach for Designing Single-Chain Magnets: Organization of Chains via Hydrogen Bonding between Nucleobases. J. Am. Chem. Soc. 2012, 134, 6908–6911. [Google Scholar] [CrossRef] [PubMed]
- Burneo, I.; Stylianou, K.C.; Rodríguez-Hermida, S.; Juanhuix, J.; Fontrodona, X.; Imaz, I.; Maspoch, D. Two New Adenine-Based Co(II) Coordination Polymers: Synthesis, Crystal Structure, Coordination Modes, and Reversible Hydrochromic Behavior. Cryst. Growth Des. 2015, 15, 3182–3189. [Google Scholar] [CrossRef]
- Wang, X.H.; Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Pei, S.; Ren, N.; Huang, L.; Huang, W. Recent developments in lanthanide-based luminescent probes. Coord. Chem. Rev. 2014, 273, 201–212. [Google Scholar] [CrossRef]
- Beobide, G.; Castillo, O.; Cepeda, J.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Thomas-Gipson, J. Metal-carboxylato-nucleobase systems: From supramolecular assemblies to 3D porous materials. Coord. Chem. Rev. 2013, 257, 2716–2736. [Google Scholar] [CrossRef]
- Givaja, G.; Amo-Ochoa, P.; Gómez-García, C.J.; Zamora, F. Electrical conductive coordination polymers. Chem. Soc. Rev. 2012, 41, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Castillo, O.; Gómez-García, C.J.; Hassanein, K.; Verma, S.; Kumar, J.; Zamora, F. Semiconductive and Magnetic One-Dimensional Coordination Polymers of Cu(II) with Modified Nucleobases. Inorg. Chem. 2013, 52, 11428–11437. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Byme, P.J.; Wheatley, P.S.; Wragg, D.S.; Zhao, X.; Fletcher, A.J.; Thomas, K.M.; Peters, L.; Evans, J.S.O.; Warren, J.E.; et al. Chemically blockable transformation and ultraselective low-pressure gas adsorption in a non-porous metal organic framework. Nat. Chem. 2009, 1, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zamora, F.; Amo-Ochoa, P.; Sanz Miguel, P.J.; Castillo, O. From metal-nucleobase chemistry towards molecular wires. Inorg. Chim. Acta 2009, 362, 691–706. [Google Scholar] [CrossRef]
- Vegas, V.G.; Lorca, R.; Latorre, A.; Hassanein, K.; Gómez-García, C.J.; Castillo, O.; Somoza, A.; Zamora, F.; Amo-Ochoa, P. Copper(II)–Thymine Coordination Polymer Nanoribbons as Potential Oligonucleotide Nanocarriers. Angew. Chem. Int. Ed. 2017, 56, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-San-Miguel, D.; Amo-Ochoa, P.; Zamora, Z. MasterChem: Cooking 2D-polymers. Chem. Commun. 2016, 52, 4113–4127. [Google Scholar] [CrossRef] [PubMed]
- Neaime, C.; Daiguebonne, C.; Calvez, G.; Freslon, S.; Bernot, K.; Grasset, F.; Cordier, S.; Guillou, O. Nanometrization of Lanthanide-Based Coordination Polymers. Chem. Eur. J. 2015, 21, 17466–17473. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, C.; Horrocks, B.R.; Martínez, J.I.; Liscio, F.; Gómez-Herrero, J.; Zamora, F. Mechanical and optical properties of ultralarge flakes of a metal-organic framework with molecular thickness. Chem. Sci. 2015, 6, 2553–2558. [Google Scholar] [CrossRef]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef]
- Liu, K.; Shen, Z.R.; Li, Y.; Han, S.D.; Hu, T.L.; Zhang, D.S.; Bu, X.H.; Ruan, W.J. Solvent induced rapid modulation of micro/nano structures of metal carboxylates coordination polymers: Mechanism and morphology dependent magnetism. Sci. Rep. 2014, 4, 6023. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, P.G.; Soleimannejad, J. Sonochemical synthesis of a new nano-sized cerium(III) supramolecular compound; Precursor for nanoceria. Ultrason. Sonochem. 2016, 31, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Wang, X.; Gao, K.; Wu, J.; Hou, H.; Fan, Y. Surfactant-Assisted Nanocrystalline Zinc Coordination Polymers: Controlled Particle Sizes and Synergistic Effects in Catalysis. Chem. Eur. J. 2016, 22, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Cacciola, M.; Ciccarella, G. A predictive model of iron oxide nanoparticles flocculation tuning Z-potential in aqueous environment for biological application. J. Nanopart. Res. 2015, 17, 377. [Google Scholar] [CrossRef]
- Nagapradeep, N.; Venkatesh, V.; Tripathi, S.K.; Verma, S. Guanine-copper coordination polymers: Crystal analysis and application as thin film precursors. Dalton Trans. 2014, 43, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Ebitani, K. Recent Advances in Heterogeneous Catalysis with Controlled Nanostructured Precious Monometals. ChemCatChem 2016, 8, 2303–2316. [Google Scholar] [CrossRef]
- Sharma, B.; Mahata, A.; Mandani, S.; Sarma, T.K.; Pathak, B. Coordination polymer hydrogels through Ag(I)-mediated spontaneous self-assembly of unsubstituted nucleobases and their antimicrobial activity. RSC Adv. 2016, 6, 62968–62973. [Google Scholar] [CrossRef]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Pérez-Yáñez, S.; Beobide, G.; Castillo, O.; Cepeda, J.; Luque, A.; Román, P. Directing the Formation of Adenine Coordination Polymers from Tunable Copper(II)/Dicarboxylato/Adenine Paddle-Wheel Building Units. Cryst. Growth Des. 2012, 12, 3324–3334. [Google Scholar] [CrossRef]
- Shi, N.E.; Du, W.; Jin, X.; Zhang, Y.; Han, M.; Xu, Z.; Xie, L.; Huang, W. Surfactant Charge Mediated Shape Control of Nano- or Microscaled Coordination Polymers: The Case of Tetrapyridylporphine Based Metal Complex. Cryst. Growth Des. 2014, 14, 1251–1257. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Usman, M.; Mendiratta, S.; Lu, K.L. Semiconductor Metal-Organic Frameworks: Future Low-Bandgap Materials. Adv. Mater. 2017, 29, 1605071. [Google Scholar] [CrossRef] [PubMed]
- Bunzen, H.; Grzywa, M.; Hambach, M.; Spirkl, S.; Volkmer, D. From Micro to Nano: A Toolbox for Tuning Crystal Size and Morphology of Benzotriazolate-Based Meta–Organic Frameworks. Cryst. Growth Des. 2016, 16, 3190–3197. [Google Scholar] [CrossRef]
- Luo, F.; Pan, C.; Cheng, J. Recent Advances in Transition-Metal-Catalyzed Esterification. Synlett 2012, 23, 357–366. [Google Scholar]
- Fu, H.; Xiao, D.; Yao, J.; Yang, G. Nanofibers of 1,3-Diphenyl-2-pyrazoline Induced by Cetyltrimethylammonium Bromide Micelles. Angew. Chem. Int. Ed. 2003, 42, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Boonmak, J.; Nakano, M.; Chaichit, N.; Pakawatchai, C.; Youngme, S. Spin Canting and Metamagnetism in 2D and 3D Cobalt(II) Coordination Networks with Alternating Double End-On and Double End-to-End Azido Bridges. Inorg. Chem. 2011, 50, 7324–7333. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Chen, Y.; Gao, J.; Wan, L.; Lei, T.; Ma, P.; Jiang, J. Synthesis, self-assembly, and semiconducting properties of phenanthroline-fused phthalocyanine derivatives. J. Mater. Chem. 2012, 22, 15695–15701. [Google Scholar] [CrossRef]









| Experiment | T (°C) | Reaction Time (min) | Adenine (M) | Cu(NO3)2·3H2O (M) | Adipic Acid (M) | Particle Size 1 (nm) |
|---|---|---|---|---|---|---|
| a | 90 | 15 | 0.010 | 0.040 | 0.040 | 1200 ± 255 |
| b | 40 | 15 | 0.015 | 0.040 | 0.040 | 381 ± 63 |
| c | 40 | 5 | 0.015 | 0.040 | 0.040 | 403 ± 130 |
| d | 40 | 5 | 0.015 | 0.050 | 0.040 | 470 ± 117 |
| e | 40 | 5 | 0.015 | 0.050 | 0.050 | 340 ± 70 |
| f | 40 | 5 | 0.040 | 0.040 | 0.040 | 521 ± 190 |
| g | 40 | 5 | 0.040 | 0.080 | 0.040 | 580 ± 107 |
| h | 40 | 5 | 0.040 | 0.040 | 0.080 | 465 ± 170 |
| Surfactant | Z-Potential (mV) | pH | Surfactant Type | Surfactant Concentration | Particle Size 1 (nm) |
|---|---|---|---|---|---|
| No surfactant | 17 | 5.8 | - | - | 403 ± 130 |
| SDS | 8 | 5.6 | Anionic | 1 mM | 422 ± 55 |
| CTAB | 42 | 6.9 | Cationic | 1 mM | 2701 ± 265 |
| P123 | 21 | 5.8 | Neutral | 1 g/L | 524 ± 212 |
| P123 | - | - | Neutral | 5 g/L | 283 ± 81 |
| P123 | - | - | Neutral | 15 g/L | 296 ± 68 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegas, V.G.; Villar-Alonso, M.; Gómez-García, C.J.; Zamora, F.; Amo-Ochoa, P. Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers 2017, 9, 565. https://doi.org/10.3390/polym9110565
Vegas VG, Villar-Alonso M, Gómez-García CJ, Zamora F, Amo-Ochoa P. Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers. 2017; 9(11):565. https://doi.org/10.3390/polym9110565
Chicago/Turabian StyleVegas, Verónica G., Marta Villar-Alonso, Carlos J. Gómez-García, Félix Zamora, and Pilar Amo-Ochoa. 2017. "Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine" Polymers 9, no. 11: 565. https://doi.org/10.3390/polym9110565
APA StyleVegas, V. G., Villar-Alonso, M., Gómez-García, C. J., Zamora, F., & Amo-Ochoa, P. (2017). Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers, 9(11), 565. https://doi.org/10.3390/polym9110565