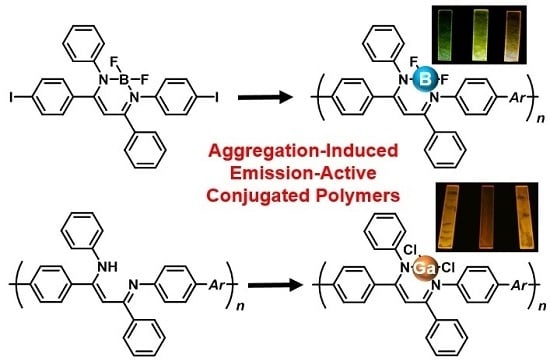
Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Conjugated Copolymers
2.2. Optical Properties
2.3. Contribution from Electronic Interaction
2.4. Electrochemical Properties of the Polymers
2.5. Density Functional Theory Calculations
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Michinobu, T. Benzothiadiazole and its π-extended, heteroannulated derivatives: Useful acceptor building blocks for high-performance donor–acceptor polymers in organic electronics. J. Mater. Chem. C 2016, 4, 6200–6214. [Google Scholar] [CrossRef]
- Parke, S.M.; Boone, M.P.; Rivard, E. Marriage of heavy main group elements with π-conjugated materials for optoelectronic applications. Chem. Commun. 2016, 52, 9485–9505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-B.; Adachi, Y.; Ooyama, Y.; Ohshita, J. Synthesis and properties of benzofuran-fused silole and germole derivatives: Reversible dimerization and crystal structures of monomers and dimers. Organometallics 2016, 35, 2327–2332. [Google Scholar] [CrossRef]
- Ooyama, Y.; Uenaka, K.; Sato, T.; Shibayama, N.; Ohshita, J. Synthesis of conjugated D–A polymers bearing bi(dithienogermole) as a new donor component and their applications to polymer solar cells and transistors. RSC Adv. 2015, 5, 12686–12691. [Google Scholar]
- Nakashima, M.; Otsura, T.; Naito, H.; Ohshita, J. Synthesis of new D–A polymers containing disilanobithiophene donor and application to bulk heterojunction polymer solar cells. Polym. J. 2015, 47, 733–738. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ueda, M.; Fukuda, K.; Fukui, K.; Takase, I.; Nishiyama, H.; Inagi, S.; Tomita, I. Synthesis of π-conjugated polymers containing phosphole units in the main chain by reaction of an organometallic polymer having a titanacyclopentadiene unit. ACS Macro Lett. 2015, 4, 124–127. [Google Scholar] [CrossRef]
- Hirose, A.; Tanaka, K.; Yoshii, R.; Chujo, Y. Film-type chemosensors based on boron diminate polymers having oxidation-induced emission properties. Polym. Chem. 2015, 6, 5590–5595. [Google Scholar] [CrossRef]
- Suenaga, K.; Yoshii, R.; Tanaka, K.; Chujo, Y. Sponge-type emissive chemosensors for the protein detection based on boron ketoiminate-modifying hydrogels with aggregation-induced blue shift emission property. Macromol. Chem. Phys. 2016, 217, 414–417. [Google Scholar] [CrossRef]
- Chujo, Y.; Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn. 2015, 88, 633–643. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015, 7, e223. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Yanagida, T.; Yamane, H.; Hirose, A.; Yoshii, R.; Chujo, Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg. Med. Chem. Lett. 2015, 25, 5331–5334. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Yamane, H.; Nagai, A.; Tanaka, K.; Taka, H.; Kita, H.; Chujo, Y. π-Conjugated polymers composed of BODIPY or Aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules 2014, 47, 2316–2323. [Google Scholar] [CrossRef]
- Yoshii, R.; Nagai, A.; Tanaka, K.; Chujo, Y. Highly NIR emissive boron di(iso)indomethene (BODIN)-based polymer: Drastic change from deep-red to NIR emission via quantitative polymer reaction. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1726–1733. [Google Scholar] [CrossRef]
- Suzuki, S.; Kozaki, M.; Nozaki, K.; Okada, K. Recent progress in controlling photophysical processes of donor–acceptor arrays involving perylene diimides and boron-dipyrromethenes. J. Photochem. Photobiol. C 2011, 12, 269–292. [Google Scholar] [CrossRef]
- Uetomo, A.; Kozaki, M.; Suzuki, S.; Yamanaka, K.; Ito, O.; Okada, K. Efficient light-harvesting antenna with a multi-porphyrin cascade. J. Am. Chem. Soc. 2011, 133, 13276–13279. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Tanaka, K.; Chujo, Y. Tunable optical property between pure red luminescence and dual-emission depended on the length of light-harvesting antennae in the dyads containing the cardo structure of BODIPY and oligofluorene. Macromolecules 2016, 49, 8899–8904. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Suenaga, K.; Tanaka, K.; Chujo, Y. Mechanofluorochromic materials based on aggregation-induced emission-active boron ketoiminates: Regulation of the direction of the emission color changes. Chem. Eur. J. 2015, 21, 7231–7237. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Nagai, A.; Tanaka, K.; Chujo, Y. Highly emissive boron ketoiminate derivatives as new class of aggregation-induced emission fluorophores. Chem. Eur. J. 2013, 19, 4506–4512. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Hirose, A.; Tanaka, K.; Chujo, Y. Boron diiminate with aggregation-induced emission and crystallization-induced emission enhancement characteristics. Chem. Eur. J. 2014, 20, 8320–8324. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Tanaka, K.; Chujo, Y. Conjugated polymers based on tautomeric units: Regulation of main-chain conjugation and expression of aggregation induced emission property via boron-complexation. Macromolecules 2014, 47, 2268–2278. [Google Scholar] [CrossRef]
- Gibson, G.L.; McCormick, T.M.; Seferos, D.S. Atomistic band gap engineering in donor–acceptor polymers. J. Am. Chem. Soc. 2012, 134, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Mahrok, A.K.; Carrera, E.I.; Tilley, A.J.; Ye, S.; Seferos, D.S. Synthesis and photophysical properties of platinum-acetylide copolymers with thiophene, selenophene and tellurophene. Chem. Commun. 2015, 51, 5475–5478. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, M.; Han, Y.; Smith, J.; Bazzi, H.S.; Alqaradawi, S.Y.A.; Watkins, S.E.; Anthopoulos, T.D.; Heeney, M. Influence of the heteroatom on the optoelectronic properties and transistor performance of soluble thiophene-, selenophene- and tellurophene-vinylene copolymers. Chem. Sci. 2016, 7, 1093–1099. [Google Scholar] [CrossRef]
- Al-Hashimi, M.; Han, Y.; Smith, J.; Bazzi, H.S.; Alqaradawi, S.Y.A.; Watkins, S.E.; Anthopoulos, T.D.; Heeney, M. An Air-Stable Semiconducting Polymer Containing Dithieno[3,2-b:2′,3′-d]arsole. Angew. Chem. Int. Ed. 2016, 55, 7148–7151. [Google Scholar]
- Matsumura, Y.; Fukuda, K.; Inagi, S.; Tomita, I. Parallel synthesis of photoluminescent π-conjugated polymers by polymer reactions of an organotitanium polymer with a titanacyclopentadiene unit. Macromol. Rapid Commun. 2015, 36, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ishidoshiro, M.; Irie, Y.; Imoto, H.; Naka, K.; Tanaka, K.; Inagi, S.; Tomita, I. Arsole-containing π-conjugated polymer by the post-element-transformation technique. Angew. Chem. Int. Ed. 2016, 55, 15040–15043. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanaka, K.; Tanaka, K.; Chujo, Y. Synthesis and characterization of heterofluorenes containing four-coordinated group 13 elements: Theoretical and experimental analyses and comparison of structures, optical properties and electronic states. Dalton Trans. 2015, 44, 8697–8707. [Google Scholar] [CrossRef]
- Ito, S.; Hirose, A.; Yamaguchi, M.; Tanaka, K.; Chujo, Y. Size-discrimination for volatile organic compounds utilizing gallium diiminate by luminescent chromism of crystallization-induced emission via encapsulation-triggered crystal-crystal transition. J. Mater. Chem. C 2016, 4, 5564–5571. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, K.; Chujo, Y. High HOMO levels and narrow energy band gaps of dithienogalloles. RSC Adv. 2015, 5, 55406–55410. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Takamine, H.; Tanaka, K.; Chujo, Y. Synthesis of air- and moisture-stable dibenzogallepins: Control of planarity of seven-membered rings in solid states by coordination to gallium atoms. Org. Lett. 2015, 17, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanaka, K.; Chujo, Y. Synthesis and characterization of gallafluorene-containing conjugated polymers: Control of emission colors and electronic effects of gallafluorene units on π-conjugation system. Macromolecules 2015, 48, 1343–1351. [Google Scholar] [CrossRef]
- Matsumoto, T.; Onishi, Y.; Tanaka, K.; Fueno, H.; Tanaka, K.; Chujo, Y. Synthesis of conjugated polymers containing gallium atoms and evaluation of conjugation though four-coordinate gallium atoms. Chem. Commun. 2014, 50, 15740–15743. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tanaka, K.; Chujo, Y. Synthesis and optical properties of stable gallafluorene derivatives: Investigation of their emission via triplet states. J. Am. Chem. Soc. 2013, 135, 4211–4214. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.; Hirose, A.; Tanaka, K.; Chujo, Y. Functionalization of boron diiminates with unique optical properties: Multicolor tuning of crystallization-induced emission and introduction into the main-chain of conjugated polymers. J. Am. Chem. Soc. 2014, 136, 18131–18139. [Google Scholar] [CrossRef] [PubMed]
- Yeo, H.; Tanaka, K.; Chujo, Y. Synthesis of dual-emissive polymers based on ineffective energy transfer through cardo fluorene-containing conjugated polymers. Polymer 2015, 60, 228–233. [Google Scholar] [CrossRef]
- Yeo, H.; Tanaka, K.; Chujo, Y. Energy transfer through heterogeneous dyes-substituted fluorene-containing alternating copolymers and their dual-emission properties. J. Polym. Sci. A Polym. Chem. 2015, 53, 2026–2035. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tamashima, K.; Nagai, A.; Okawa, T.; Chujo, Y. Facile modulation of optical properties of diketonate-containing polymers by regulating complexation ratios with boron. Macromolecules 2013, 46, 2969–2975. [Google Scholar] [CrossRef]
- Gibson, G.L.; McCormick, T.M.; Seferos, D.S. Effect of group-14 and group-16 substitution on the photophysics of structurally related donor–acceptor polymers. J. Phys. Chem. C 2013, 117, 16606–16615. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Jiang, T.; Wu, L.-B.; Wan, J.-H.; Chen, C.-H.; Pei, Y.-B.; Lu, H.; Deng, Y.; Bian, G.-F.; Qiu, H.-Y.; et al. 2,3,4,5-Tetraphenylsilole-based conjugated polymers: Synthesis, optical properties, and as sensors for explosive compounds. Chem. Asian J. 2012, 7, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Kulkarni, A.P.; Wu, P.-T.; Kwon, T.W.; Jenekhe, S.A. Phenothiazine-phenylquinoline donor–acceptor molecules: Effects of structural isomerism on charge transfer photophysics and electroluminescence. J. Phys. Chem. B 2005, 109, 19584–19594. [Google Scholar] [CrossRef]
- Filarowski, A.; Kluba, M.; Cieślik-Boczula, K.; Koll, A.; Kochel, A.; Pandey, L.; De Borggraeve, W.M.; Van der Auweraer, M.; Catalán, J.; Boens, N. Generalized solvent scales as a tool for investigating solvent dependence of spectroscopic and kinetic parameters. Application to fluorescent BODIPY dyes. Photochem. Photobiol. Sci. 2010, 9, 996–1008. [Google Scholar]
- Pina, J.; de Melo, J.S.; Breusov, D.; Scherf, U. Donor–acceptor–donor thienyl/bithienyl-benzothiadiazole/quinoxaline model oligomers: Experimental and the oretical studies. Phys. Chem. Chem. Phys. 2013, 15, 15204–15213. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.L.; Seferos, D.S. “Heavy-atom” donor–acceptor conjugated polymers. Macromol. Chem. Phys. 2014, 215, 811–823. [Google Scholar] [CrossRef]
- Thirion, D.; Rault-Berthelot, J.; Vignau, L.; Poriel, C. Synthesis and properties of a blue bipolar indenofluorene emitter based on a D-π-A design. Org. Lett. 2011, 13, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.E.; Fisher, A.L.; Pearson, C.; Fox, M.A.; Pålsson, L.-O.; Bryce, M.R.; Petty, M.C. Colour tuning of blue electroluminescence using bipolar carbazole–oxadiazole molecules in single-active-layer organic light emitting devices (OLEDs). J. Mater. Chem. 2012, 22, 11816–11825. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ford, M.J.; Lill, A.T.; Phan, H.; Nguyen, T.-Q.; Bazan, G.C. Hole mobility and electron injection properties of D-A conjugated copolymers with fluorinated phenylene acceptor units. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Tonzola, C.J.; Babel, A.; Jenekhe, S.A. Electron transport materials for organic light-emitting diodes. Chem. Mater. 2004, 16, 4556–4573. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]





| Polymer | Mn | Mw | Mw/Mn | n b |
|---|---|---|---|---|
| BF | 13,500 | 34,000 | 2.5 | 15 |
| BC | 15,200 | 38,000 | 2.5 | 13 |
| BT | 12,200 | 28,800 | 2.4 | 18 |
| LF | 9200 | 27,000 | 2.9 | 11 |
| LC | 7000 | 15,400 | 2.2 | 9.0 |
| LT | 7100 | 14,000 | 2.0 | 8.1 |
| GaF | 9400 | 18,000 | 1.9 | 9.4 |
| GaC | 10,000 | 18,000 | 1.8 | 11 |
| GaT | 8800 | 14,000 | 1.6 | 8.7 |
| Polymer | λabssolution/nm | λabsfilm/nm | ε × 104/M−1·cm−1 a | λPLsolution/nm b | λPLfilm/nm c | ΦPLfilm c,d |
|---|---|---|---|---|---|---|
| BF | 399 | 399 | 5.99 | 545 | 545 | 0.07 |
| BC | 397 | 404 | 4.00 | 545 | 552 | 0.07 |
| BT | 404 | 417 | 4.96 | 581 | 575 | 0.07 |
| GF | 411 | 415 | 3.94 | 571 | 575 | 0.05 |
| GC | 410 | 414 | 3.91 | 576 | 573 | 0.05 |
| GT | 420 | 424 | 3.83 | 610 | 601 | 0.03 |
| Polymer | λonset/nm a | Egopt/eV b | EredCV/V c,d | EHOMO/eV e | ELUMO/eV f |
|---|---|---|---|---|---|
| BF | 451 | 2.74 | −1.27 | −6.27 | −3.53 |
| BC | 452 | 2.74 | −1.36 | −6.18 | −3.44 |
| BT | 467 | 2.65 | −1.36 | −6.10 | −3.45 |
| GaF | 479 | 2.59 | −1.20 | −6.19 | −3.60 |
| GaC | 475 | 2.61 | −1.26 | −6.15 | −3.54 |
| GaT | 488 | 2.54 | −1.26 | −6.08 | −3.54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, S.; Hirose, A.; Yamaguchi, M.; Tanaka, K.; Chujo, Y. Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element. Polymers 2017, 9, 68. https://doi.org/10.3390/polym9020068
Ito S, Hirose A, Yamaguchi M, Tanaka K, Chujo Y. Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element. Polymers. 2017; 9(2):68. https://doi.org/10.3390/polym9020068
Chicago/Turabian StyleIto, Shunichiro, Amane Hirose, Madoka Yamaguchi, Kazuo Tanaka, and Yoshiki Chujo. 2017. "Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element" Polymers 9, no. 2: 68. https://doi.org/10.3390/polym9020068
APA StyleIto, S., Hirose, A., Yamaguchi, M., Tanaka, K., & Chujo, Y. (2017). Synthesis of Aggregation-Induced Emission-Active Conjugated Polymers Composed of Group 13 Diiminate Complexes with Tunable Energy Levels via Alteration of Central Element. Polymers, 9(2), 68. https://doi.org/10.3390/polym9020068