The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Samples
2.1.1. Samples for DMU-Formaldehyde (DMUF) Reactions under Alkaline and Weak Acidic Conditions
2.1.2. Samples for Melamine-Formaldehyde (MF) Reactions under Weak Acidic Conditions
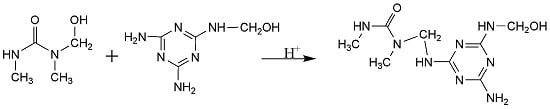
2.1.3. Samples for Melamine-DMU-Formaldehyde (MDMUF) Reactions under Alkaline and Weak Acidic Conditions
2.2. 13C-NMR Characterizations
3. Results and Discussion
3.1. The DMU-Formaldehyde (DMUF) Reactions
3.2. The Melamine-Formaldehyde (MF) Reactions
3.3. The Melamine-DMU-Formaldehyde (MDMUF) Reactions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunky, M. Adhesives based on formaldehyde condensation resins. Macromol. Symp. 2004, 217, 417–429. [Google Scholar] [CrossRef]
- Tomita, B.; Hse, C.Y. Analysis on cocondensation of melamine and urea through carbon 13 enriched formaldehyde with carbon 13 nuclear magnetic resonance spectroscopy. Mokuzai Gakkaishi 1995, 41, 490–497. [Google Scholar]
- Tomita, B.; Hse, C.Y. Analyses of cocondensation of melamine and urea through formaldehyde with carbon 13 nuclear magnetic resonance spectroscopy. Mokuzai Gakkaishi 1995, 41, 349–354. [Google Scholar]
- No, B.Y.; Kim, M.G. Syntheses and properties of low-level melamine-modified urea-melamine-formaldehyde resins. J. Appl. Polym. Sci. 2004, 93, 2559–2569. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Kim, H.; Lee, Y.; Yang, H. Thermal analysis study of viscoelastic properties and activation energy of melamine-modified urea-formaldehyde resins. J. Adhes. Sci. Technol. 2006, 20, 803–816. [Google Scholar] [CrossRef]
- No, B.Y.; Kim, M.G. Evaluation of melamine-modified urea-formaldehyde resins as particleboard binders. J. Appl. Polym. Sci. 2007, 106, 4148–4156. [Google Scholar]
- Hse, C.Y.; Fu, F.; Pan, H. Melamine-modified urea formaldehyde resin for bonding particleboards. For. Prod. J. 2008, 58, 56–61. [Google Scholar]
- Park, B.D.; Lee, S.M.; Roh, J.K. Effects of formaldehyde/urea mole ratio and melamine content on the hydrolytic of cured urea-melamine-formaldehyde resin. Eur. J. Wood Prod. 2009, 57, 121–123. [Google Scholar] [CrossRef]
- Sun, Q.; Hse, C.Y.; Shupe, T.F. Characterization and performance of melamine enhanced urea formaldehyde resin for bonding southern pine. J. Appl. Polym. Sci. 2011, 119, 3538–3543. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Gaun, L.T.; Sun, J.; Tu, D.Y. Preparation and characteristic of urea formaldehyde modified with hexamethoxyl melamine. Adv. Mater. Res. 2011, 160–162, 1245–1252. [Google Scholar]
- Paiva, N.T.; Henriques, A.; Cruz, P.; Ferra, J.M.; Carvalho, L.H.; Magalhães, F.D. Production of melamine fortified urea-formaldehyde resins with low formaldehyde emission. J. Appl. Polym. Sci. 2012, 124, 2311–2317. [Google Scholar] [CrossRef]
- Paiva, N.T.; Pereira, J.; Ferra, J.M.; Cruz, P.; Carvalho, L.; Magalhães, F.D. Study of influence of synthesis conditions on properties of melamine–urea formaldehyde resins. Int. Wood Prod. J. 2012, 3, 51–57. [Google Scholar] [CrossRef]
- Tohmura, S.; Inoue, A.; Sahari, S.H. Influence of the melamine content in melamine-urea-formaldehyde resins on formaldehyde emission and cured resin structure. J. Wood Sci. 2001, 47, 451–457. [Google Scholar] [CrossRef]
- No, B.Y.; Kim, M.G. Curing of low level melamine-modified urea-formaldehyde particleboard binder resins studied with dynamic mechanical analysis (DMA). J. Appl. Polym. Sci. 2005, 97, 377–389. [Google Scholar]
- Kandelbauer, A.; Despres, A.; Pizzi, A.; Taudes, I. Testing by fourier transform infrared species variation during melamine–urea–formaldehyde resin preparation. J. Appl. Polym. Sci. 2007, 106, 2192–2197. [Google Scholar] [CrossRef]
- Despres, A.; Pizzi, A.; Pasch, H.; Kandelbauer, A. Comparative 13C-NMR and matrix-assisted laser desorption/ionization time-of-flight analyses of species variation and structure maintenance during melamine–urea–formaldehyde resin preparation. J. Appl. Polym. Sci. 2007, 106, 1106–1128. [Google Scholar] [CrossRef]
- Siimer, K.; Christjanson, P.; Kaljuvee, T.; Pehk, T.; Lasn, I.; Saks, I. TG-DTA study of melamine-urea-formaldehyde resins. J. Therm. Anal. Calorim. 2008, 92, 19–27. [Google Scholar] [CrossRef]
- Siimer, K.; Kaljuvee, T.; Pehk, T.; Lasn, I. Thermal behaviour of melamine-modified urea–formaldehyde resins. J. Therm. Anal. Calorim. 2010, 99, 755–762. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Petek, P.; Modved, S.; Pizzi, A. On the performance of a melamine-urea-formaldehyde resin for decorative paper coatings. Eur. J. Wood Prod. 2010, 68, 63–75. [Google Scholar] [CrossRef]
- Philbrook, A.; Blake, C.; Dunlop, J.N.; Easton, C.J.; Keniry, M.A. Demonstration of co-polymerization in melamine–urea–formaldehyde reactions using 15N NMR correlation spectroscopy. Polymer 2005, 46, 2153–2156. [Google Scholar] [CrossRef]
- Steinhof, O.; Scherrb, G.; Hasse, H. Investigation of the reaction of 1,3-dimethylurea with formaldehyde by quantitative on-line NMR spectroscopy: A model for the urea–formaldehyde system. Magn. Reson. Chem. 2016, 54, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Siimer, K.; Pehk, T.; Christjanson, P. Study of the structural changes in urea-formaldehyde condensates during synthesis. Macromol. Symp. 1999, 148, 149–156. [Google Scholar] [CrossRef]
- Li, T.H.; Du, G.B.; Guo, X.S.; Liang, J.K.; Wang, H.; Xie, X.G. Competitive Formation of the Methylene and Methylene ether Bridges in the Urea-formaldehyde Reaction in Alkaline Solution: A Combined Experimental and Theoretical Study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar] [CrossRef]
- Li, T.H. Study on the Mechanisms of the Reactions in Synthesis of Amino Resins Used as Wood Adhesives. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2015. [Google Scholar]
- Li, T.H.; Liang, J.H.; Cao, M.; Guo, X.S.; Xie, X.G.; Du, G.B. Re-elucidation of the acid-catalyzed urea–formaldehyde reactions: A theoretical and 13C-NMR study. J. Appl. Polym. Sci. 2016, 133, 44339–44356. [Google Scholar] [CrossRef]








| F:DMU = 1:1 | F:M = 2:1 | |||||
|---|---|---|---|---|---|---|
| Structures | Chemical shift (ppm) | A1 | A2 | Structures | Chemical shift (ppm) | B |
| pH = 9 | pH = 6 | pH = 6 | ||||
| –N(CH3)–CH2–N(CH3)– | 61–62 | — | 9.7 | –NH–CH2–NH– (I) | 47–48 | 5.6 |
| Triazine(–CH2–) | 68–70 | 1.9 | 1.1 | –NH–CH2–N= (II) | 54–55 | 2.7 |
| H3C–NH–CO–N(–CH3)–CH2OH | 71–73 | 77.2 | 50.6 | =N–CH2–N= (III) | 60–61 | —— |
| Total | 8.3 | |||||
| –N(CH3) –CH2–O–CH2–N(CH3)- –N(–CH3)–CH2OCH2OH | 77–78 | 4.6 | 13.5 | |||
| –NH–CH2OCH2NH– (I) | 68–70 | 1.0 | ||||
| –NH–CH2OCH2N= (II) | 77–78 | —— | ||||
| Uron(–CH2–O–CH2–) –N(–CH3)CH2OCH3 | 80–81 | — | 5.3 | |||
| Total | 1.0 | |||||
| Total | 83.7 | 80.2 | –NH–CH2OH (I) | 64–66 | 69.3 | |
| HO–CH2–OH | 82–84 | 4.4 | 3.8 | –N(–CH2)–CH2OH (II) | 71–73 | 17.1 |
| HOCH2–O–CH2–OCH2OH –N(–CH3)–CH2–O–CH2OH | 86–88 | 4.4 | 7.1 | Total | 86.4 | |
| HO–CH2–OH | 82–84 | 1.3 | ||||
| HOCH2-O–CH2–OCH2OH | 90–91 | 7.5 | 8.2 | HOCH2-O–CH2–OCH2OH –NH–CH2–O–CH2OH | 86–88 | 0.6 |
| H(CH2O)nOCH2OCH3 | 93–95 | — | 0.7 | HOCH2–O–CH2–OCH2OH | 90–91 | 1.3 |
| Total | 16.3 | 19.8 | H(CH2O)nO–CH2OCH3 | 93–95 | —— | |
| Total | 3.2 | |||||
| –NH–CH2–O–CH3 | 73–74 | 1.1 | ||||
| Structures | Chemical Shift (ppm) | C1 | C2 | Structures | Chemical Shift (ppm) | C1 | C2 |
|---|---|---|---|---|---|---|---|
| pH = 9 | pH = 6 | pH = 9 | pH = 6 | ||||
| –NH–CH2–NH– (I) | 47–48 | —— | 12.8 | –NH–CH2OH (I) | 64–66 | 44.3 | 9.7 |
| 54–55 | —— | 46.5 | 71–73 | 25.6 | 2.8 | ||
| –NH–CH2–N= (II) | –N(–CH2) –CH2OH (II) | ||||||
| –NH–CH2–N(–CH3)– | –N(–CH3) –CH2OH | ||||||
| 61–62 | —— | 23.1 | Total | 69.8 | 12.5 | ||
| =N–CH2–N= (III) | |||||||
| –N(–CH3) –CH2–N(–CH3)- | HO–CH2–OH | 82–84 | 0.4 | 0.1 | |||
| Total | 0 | 82.4 | 86–88 | —— | —— | ||
| HOCH2–O–CH2–OCH2OH | |||||||
| –NH–CH2OCH2NH– (I) | 68–70 | 22.8 | 2.4 | ||||
| –N(–CH3) –CH2OCH2OH | |||||||
| –NH–CH2OCH2N= (II) | 76–78 | 4.0 | —— | HOCH2–O–CH2–OCH2OH | 89–91 | 1.1 | 0.3 |
| =N–CH2OCH2N= (III) | H(CH2O)nO–CH2OCH3 | 93–95 | —— | —— | |||
| –N(–CH3)–CH2OCH2–N(-CH3) | Total | 1.5 | 0.4 | ||||
| Uron(–CH2–O–CH2–) | 78–80 | —— | 2.3 | –NH–CH2–O–CH3 | 73–74 | 1.9 | —— |
| –NH(–CH3) –CH2OCH3 | |||||||
| Total | 26.8 | 4.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Li, T.; Liang, J.; Du, G. The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study. Polymers 2017, 9, 109. https://doi.org/10.3390/polym9030109
Cao M, Li T, Liang J, Du G. The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study. Polymers. 2017; 9(3):109. https://doi.org/10.3390/polym9030109
Chicago/Turabian StyleCao, Ming, Taohong Li, Jiankun Liang, and Guanben Du. 2017. "The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study" Polymers 9, no. 3: 109. https://doi.org/10.3390/polym9030109
APA StyleCao, M., Li, T., Liang, J., & Du, G. (2017). The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study. Polymers, 9(3), 109. https://doi.org/10.3390/polym9030109