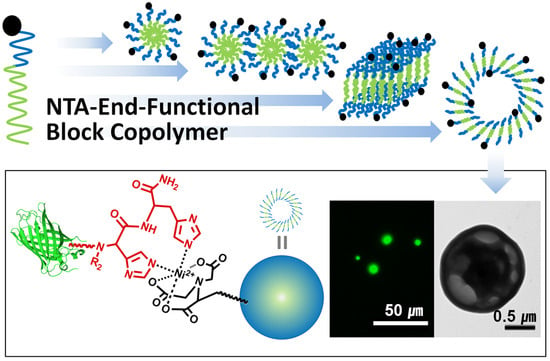
Morphology Control of Ni(II)-NTA-End-Functionalized Block Copolymer and Bio-Conjugation through Metal-Ligand Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of t-boc-NTA-End-Functionalized p(PEGMA)
2.4. Synthesis of t-boc-NTA-p(PEGMA-b-St) Block Copolymer (3)
2.5. Synthesis of Ni2+-NTA-p(PEGMA-b-St) (5)
2.6. Self-Assembly of Block Copolymer
2.7. Bioconjugation of Polymeric Vesicles with His6-GFP
2.8. Release of His6-GFP from the Hybrid
3. Results and Discussion
3.1. Synthesis Nitrilotiracetic Acid (NTA)-Functionalized Block Copolymer
3.2. Morphology Transition of Block Copolymer via Self-Assembly in THF/Water
3.3. Bioconjugation with His6-GFP
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klok, H.A.; Lecommandoux, S. Supramolecular materials via block copolymer self-assembly. Adv. Mater. 2001, 13, 1217–1229. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Ikkala, O.; ten Brinke, G. Functional materials based on self-assembly of polymeric supramolecules. Science 2002, 295, 2407–2409. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Formation of crew-cut aggregates of various morphologies from amphiphilic block copolymers in solution. Polym. Adv. Technol. 1998, 9, 677–699. [Google Scholar] [CrossRef]
- Joralemon, M.J.; Smith, N.L.; Holowka, D.; Baird, B.; Wooley, K.L. Antigen-decorated shell cross-linked nanoparticles: Synthesis, characterization, and antibody interactions. Bioconjug. Chem. 2005, 16, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Metera, K.; Sleiman, H.F. Biotin-terminated ruthenium bipyridine ring-opening metathesis polymerization copolymers: Synthesis and self-assembly with streptavidin. Macromolecules 2005, 38, 1084–1090. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Joralemon, M.J.; Hawker, C.J.; Wooley, K.L. Facile syntheses of surface-functionalized micelles and shell cross-linked nanoparticles. J. Polym. Sci. Part A 2006, 44, 5203–5217. [Google Scholar] [CrossRef]
- Shi, M.; Wosnick, J.H.; Ho, K.; Keating, A.; Shoichet, M.S. Immuno-polymeric nanoparticles by diels–alder chemistry. Angew. Chem. Int. Ed. 2007, 46, 6126–6131. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Gondi, S.R.; Sumerlin, B.S. Folate-conjugated thermoresponsive block copolymers: Highly efficient conjugation and solution self-assembly. Biomacromolecules 2008, 9, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Nehring, R.; Palivan, C.G.; Casse, O.; Tanner, P.; Tuxen, J.; Meier, W. Amphiphilic diblock copolymers for molecular recognition: Metal-nitrilotriacetic acid functionalized vesicles. Langmuir 2009, 25, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Vyhnalkova, R.; Mueller, A.H.; Eisenberg, A. Control of morphology and corona composition in aggregates of mixtures of ps-b-paa and ps-b-p4vp diblock copolymers: Effects of solvent, water content and mixture composition. Langmuir 2014, 30, 13152–13163. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Schmidt, V.; Aissou, K.; Borsali, R. Block copolymer systems: From single chain to self-assembled nanostructures. Langmuir 2010, 26, 15734–15744. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-D.; Li, G.L.; Neoh, K.; Kang, E. Hollow polymeric nanostructures—Synthesis, morphology and function. Prog. Polym. Sci. 2011, 36, 127–167. [Google Scholar] [CrossRef]
- Choucair, A.; Eisenberg, A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur. Phys. J. E Soft Matter 2003, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Zheng, J.X.; Li, P.; Quirk, R.P.; Harris, F.W.; Cheng, S.Z.D. Self-assembled polystyrene-block-poly(ethylene oxide) micelle morphologies in solution. Macromolecules 2006, 39, 4880–4888. [Google Scholar] [CrossRef]
- Bhargava, P.; Tu, Y.; Zheng, J.X.; Xiong, H.; Quirk, R.P.; Cheng, S.Z.D. Temperature-induced reversible morphological changes of polystyrene-block-poly(ethylene oxide) micelles in solution. J. Am. Chem. Soc. 2007, 129, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Kapanidis, A.N.; Ebright, Y.W.; Ebright, R.H. Site-specific incorporation of fluorescent probes into protein: Hexahistidine-tag-mediated fluorescent labeling with (ni2+: Nitrilotriacetic acid) n-fluorochrome conjugates. J. Am. Chem. Soc. 2001, 123, 12123–12125. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.A.; Lee, C.; Han, H.S.; Kim, B.-S.; Ha, E.-J.; Jeong, J.; Song, J.K.; Lee, S.-G.; An, S.S.A.; Paik, H.-J. In situ formation of polymer–protein hybrid spherical aggregates from (nitrilotriacetic acid)-end-functionalized polystyrenes and his-tagged proteins. Polym. Chem. 2013, 4, 2286–2292. [Google Scholar] [CrossRef]
- Andreescu, S.; Magearu, V.; Lougarre, A.; Fournier, D.; Marty, J.-L. Immobilization of enzymes on screen-printed sensors via an histidine tail. Application to the detection of pesticides using modified cholinesterase. Anal. Lett. 2001, 34, 529–540. [Google Scholar] [CrossRef]
- Altin, J.G.; White, F.A.; Easton, C.J. Synthesis of the chelator lipid nitrilotriacetic acid ditetradecylamine (nta-dtda) and its use with the iasys biosensor to study receptor–ligand interactions on model membranes. Biochim. Biophys. Acta Biomembr. 2001, 1513, 131–148. [Google Scholar] [CrossRef]
- Porath, J.; Carlsson, J.; Olsson, I.; Belfrage, G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 1975, 258, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Gaberc-Porekar, V.; Menart, V. Potential for using histidine tags in purification of proteins at large scale. Chem. Eng. Technol. 2005, 28, 1306–1314. [Google Scholar] [CrossRef]
- Crowe, J.; Dobeli, H.; Gentz, R.; Hochuli, E.; Stiiber, D.; Henco, K. 6xffis-ni-nta chromatography as a superior technique in recombinant protein expression/purification. Methods Mol. Biol. 1994, 31, 371–387. [Google Scholar] [PubMed]
- He, X.-M.; Zhu, G.-T.; Lu, W.; Yuan, B.-F.; Wang, H.; Feng, Y.-Q. Nickel (ii)-immobilized sulfhydryl cotton fiber for selective binding and rapid separation of histidine-tagged proteins. J. Chromatogr. A 2015, 1405, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.A.; Kim, S.J.; Ha, E.J.; Cho, H.Y.; Kim, B.S.; Choi, D.; Lee, S.G.; Kim, B.G.; Kim, S.W.; Paik, H.J. Encapsulation of nanoparticles using nitrilotriacetic acid end-functionalized polystyrenes and their application for the separation of proteins. Adv. Funct. Mater. 2012, 22, 4032–4037. [Google Scholar] [CrossRef]
- Ueda, E.; Gout, P.; Morganti, L. Current and prospective applications of metal ion–protein binding. J. Chromatogr. A 2003, 988, 1–23. [Google Scholar] [CrossRef]
- Chebil, S.; Macauley, N.; Hianik, T.; Korri-Youssoufi, H. Multiwalled carbon nanotubes modified by nta-copper complex for label-free electrochemical immunosensor detection. Electroanalysis 2013, 25, 636–643. [Google Scholar] [CrossRef]
- Chikh, G.G.; Li, W.M.; Schutze-Redelmeier, M.-P.; Meunier, J.-C.; Bally, M.B. Attaching histidine-tagged peptides and proteins to lipid-based carriers through use of metal-ion-chelating lipids. Biochim. Biophys. Acta Biomembr. 2002, 1567, 204–212. [Google Scholar] [CrossRef]
- Platt, V.; Huang, Z.; Cao, L.; Tiffany, M.; Riviere, K.; Szoka, F.C., Jr. Influence of multivalent nitrilotriacetic acid lipid−ligand affinity on the circulation half-life in mice of a liposome-attached his6-protein. Bioconjug. Chem. 2010, 21, 892–902. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Piehler, J. Multivalent chelators for spatially and temporally controlled protein functionalization. Anal. Bioanal. Chem. 2014, 406, 3345–3357. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kadir, M.A.; Kim, B.-S.; Han, H.S.; Nagasundarapandian, S.; Kim, Y.-R.; Ko, S.B.; Lee, S.-G.; Paik, H.-J. Synthesis of well-defined (nitrilotriacetic acid)-end-functionalized polystyrenes and their bioconjugation with histidine-tagged green fluorescent proteins. Macromolecules 2011, 44, 4672–4680. [Google Scholar] [CrossRef]
- Kadir, M.A.; Park, J.H.; Lee, J.; Lee, C.; Lee, S.H.; Lee, S.G.; Paik, H.J. Multivalent (nitrilotriacetic acid)-end-functionalized polystyrenes by atrp and their self-assembly. Macromol. Chem. Phys. 2013, 214, 2027–2035. [Google Scholar] [CrossRef]
- Davis, K.A.; Charleux, B.; Matyjaszewski, K. Preparation of block copolymers of polystyrene and poly (t-butyl acrylate) of various molecular weights and architectures by atom transfer radical polymerization. J. Polym. Sci. Part A 2000, 38, 2274–2283. [Google Scholar] [CrossRef]
- Shinoda, H.; Matyjaszewski, K. Structural control of poly (methyl methacrylate)-g-poly (lactic acid) graft copolymers by atom transfer radical polymerization (atrp). Macromolecules 2001, 34, 6243–6248. [Google Scholar] [CrossRef]
- Ziegler, M.J.; Matyjaszewski, K. Atom transfer radical copolymerization of methyl methacrylate and n-butyl acrylate. Macromolecules 2001, 34, 415–424. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Rettig, H.; Krause, E.; Börner, H.G. Atom transfer radical polymerization with polypeptide initiators: A general approach to block copolymers of sequence-defined polypeptides and synthetic polymers. Macromol. Rapid Commun. 2004, 25, 1251–1256. [Google Scholar] [CrossRef]
- Ayres, L.; Adams, P.H.H.; Löwik, D.W.; van Hest, J.C. Β-sheet side chain polymers synthesized by atom-transfer radical polymerization. Biomacromolecules 2005, 6, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Shipp, D.A.; Wang, J.-L.; Matyjaszewski, K. Synthesis of acrylate and methacrylate block copolymers using atom transfer radical polymerization. Macromolecules 1998, 31, 8005–8008. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Shipp, D.A.; Wang, J.-L.; Grimaud, T.; Patten, T.E. Utilizing halide exchange to improve control of atom transfer radical polymerization. Macromolecules 1998, 31, 6836–6840. [Google Scholar] [CrossRef]
- Bhargava, P.; Zheng, J.X.; Quirk, R.P.; Cheng, S.Z. Self-assembled, wormlike-cylinder network of polystyrene-block-poly (ethylene oxide) micelles in solution. J. Polym. Sci. Part B 2006, 44, 3605–3611. [Google Scholar] [CrossRef]
- Lim Soo, P.; Eisenberg, A. Preparation of block copolymer vesicles in solution. J. Polym. Sci. Part B 2004, 42, 923–938. [Google Scholar] [CrossRef]
- Esser-Kahn, A.P.; Francis, M.B. Protein-cross-linked polymeric materials through site-selective bioconjugation. Angew. Chem. 2008, 120, 3811–3814. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Lee, C.; Chae, M.; Kadir, M.A.; Choi, J.E.; Song, J.K.; Paik, H.-j. Morphology Control of Ni(II)-NTA-End-Functionalized Block Copolymer and Bio-Conjugation through Metal-Ligand Complex. Polymers 2017, 9, 144. https://doi.org/10.3390/polym9040144
Park D, Lee C, Chae M, Kadir MA, Choi JE, Song JK, Paik H-j. Morphology Control of Ni(II)-NTA-End-Functionalized Block Copolymer and Bio-Conjugation through Metal-Ligand Complex. Polymers. 2017; 9(4):144. https://doi.org/10.3390/polym9040144
Chicago/Turabian StylePark, Dasom, Chaeyeon Lee, Minsu Chae, Mohammad Abdul Kadir, Ji Eun Choi, Jae Kwang Song, and Hyun-jong Paik. 2017. "Morphology Control of Ni(II)-NTA-End-Functionalized Block Copolymer and Bio-Conjugation through Metal-Ligand Complex" Polymers 9, no. 4: 144. https://doi.org/10.3390/polym9040144
APA StylePark, D., Lee, C., Chae, M., Kadir, M. A., Choi, J. E., Song, J. K., & Paik, H. -j. (2017). Morphology Control of Ni(II)-NTA-End-Functionalized Block Copolymer and Bio-Conjugation through Metal-Ligand Complex. Polymers, 9(4), 144. https://doi.org/10.3390/polym9040144